Abstract
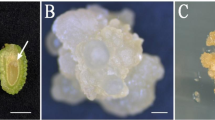
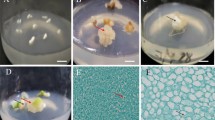
A range of tissue culture conditions were tested to improve embryo culture frequency, and to develop an efficient plant regeneration system for triticale. Immature embryos (14–21 days post-anthesis) from two triticale genotypes (Hx87-139 and Tahara) were cultured on a commonly used Murashige and Skoog (MS) and on Lazzeri's (L1) basal medium with varied carbon sources, and two different plant growth regulators; 2,4-Dichlorophenoxyacetic acid (2,4-D) and 3,6-Dichloro-2-methoxybenzoic acid (dicamba). Although embryos could be cultured on both media types, L1 based medium was better than MS basal salts for callus induction and somatic embryogenesis, with plant regeneration frequencies up to 11 fold greater on L1 media types. In the presence of dicamba, callus induction was more rapid, that resulted in subsequent regeneration of up to 2 fold more plantlets than from callus induced on medium containing 2,4-D. Maltose appeared to be a superior carbon source during differentiation of callus. Genotype Tahara showed a better regenerative response than Hx87-138, with up to 23 normal, fertile plants being produced from a single embryo when cultured on L1MDic medium, containing maltose (5%) and dicamba (20 mg l−1). Applications of this tissue culture procedure in triticale improvement through genetic engineering are also discussed.
Similar content being viewed by others
References
Claparols I, Santos MA and Torne JM(1993) Influence of some exogenous amino acids on the production of maize embryogenic callus and on endogenous amino acid content. Plant Cell Tissue Organ Cult 34: 1–11
Eapen S and Rao PS (1982) Callus induction and plant regeneration from immature embryos of rye and triticale. Plant Cell Tissue Organ Cult 1: 221–227
Felfoldi K and Purnhauser L (1992) Induction of regenerating callus cultures from immature embryos of 44 wheat and 3 triticale cultivars. Cereal Res Commun 20: 273–277
Gamborg OL (1970) The effects of amino acids and ammonium on the growth of plant cells in suspension cultures. Plant Physiol 45: 372–373
Grimes HD and Hodges TK (1990) The inorganic NO3-:NH4+ ratio influences plant regeneration and auxin sensitivity in primary callus derived from immature embryos of indica rice (Oryza sativa L.) J Plant Physiol 136: 362–367
Gustafson JP, Bushuck W and Dera AR (1991) Triticale: Production and Utilisation. In: Lorenz KJ and Kulp K (eds) Handbook of Cereal Science and Technology, pp 373–399, New York: Marcel Dekker Incorporated
Hesemann CU (1990) Application of tissue and cell culture techniques in developing productive triticale. In: Proceedings of the Second International Triticale Symposium, Passo Fundo, Brazil, pp 295–297, Mexico, D.F.: CIMMYT
Hossain MT and Islam AS (1993) In vitro plant regeneration in triticale and wheat. Bangladesh J Sci Ind Res 28: 17–24
Hunsinger H and Schauz K (1987) The influence of dicamba on somatic embryogenesis and frequency of plant regeneration from cultured immature embryos of wheat (Triticum aestivum L.) Plant Breed 98: 119–123
Immonen AST and Varughese G (1990) Use of callus culture to facilitate production of primary triticales. In: Proceedings of the Second International Triticale Symposium, Passo Fundo, Brazil, pp 381–382, Mexico, D.F.: CIMMYT
Immonen AST (1992) Effect of karyotype on somatic embryogenesis from immature triticale embryos (X Triticosecale Wittmack). Plant Breed 109: 116–122
Immonen AST (1993) Amino acid medium for somatic embryogenesis from immature triticale (X Triticosecale Wittmack) embryos. Cereal Res Commun 21: 51–55
Immonen AST (1996) Influence of media and growth regulators on somatic embryogenesis and plant regeneration for production of primary triticales. Plant Cell Tissue Organ Cult 44: 45–52
Kapila RK and Sethi GS (1993) Genotype and age effect on in vitro embryo rescue of bread wheat X hexaploid triticale hybrids. Plant Cell Tissue Organ Cult 35: 287–291
Karsai I, Bedo Z and Hayes PM (1994) Effect of induction medium pH and maltose concentration on in vitro androgenesis of hexaploid winter triticale and wheat. Plant Cell TissueOrgan Cult 39: 49–53
Lazzeri PA, Jahne A and Lorz H (1991) Culture, regeneration and transformation of barley protoplasts. In: Lindsey K (ed), Plant Tissue Culture Manual, Germany: Kluwer Academic
Millam S, Davidson D and Powell W (1992) The use of flax (Linum usitatissimum) as amodel system for studies on organogenesis in vitro: the effect of different carbohydrates. Plant Cell Tissue Organ Cult 28: 163–166
Murashige T and Skoog F (1962) A revised medium for rapid growth and bioassayswith tobacco tissue cultures. Physio Plant 15: 473–497
Nakamura C and Keller WA (1982) Callus proliferation and plant regeneration from immature embryos of hexaploid triticale. Zeitschrift-fur-Pflanzenzuchtung 88: 137–160
Papenfuss JM and Carman JG (1987) Enhanced regeneration from wheat callus cultures using dicamba and kinetin. Crop Sci 27: 588–593
Scott P and Lyne RL (1994) The effect of different carbohydrate sources upon the initiation of embryogenesis from barley microspores. Plant Cell Tissue Organ Cult 36: 129–133
Sharma GC, Bello LL and Sapra VT (1980) Genotypic differences in organogenesis from callus of ten triticale lines. Euphytica 29: 751–754
Sirkka A and Immonen T (1993) Comparison of callus culture with embryo culture at different times of embryo rescue for primary triticale production. Euphytica 70: 185–190
Stolarz A and Lorz H (1986) Somatic embryogenesis, in vitro multiplication and plant regeneration from immature embryo explants of hexaploid triticale (x Triticosecale Wittmack). Zeitschrift-fur-Pflanzenzuchtung 96: 353–362
Varughese G, Pfeifer WH and Pena RJ (1996) Triticale: A successful alternative crop (part 1). Cereals Foods World 41: 474–482
Zimny J, Becker D, Brettschneider R and Lorz H (1995) Fertile, transgenic Triticale (x Triticosecale Wittmack). Molecular Breeding 1: 155–164
Zhu M, Xu A, Yuan M, Huang C, Yu Z, Wang L and Yu J (1990) Effects of amino acids on callus differentiation in barley anther culture. Plant Cell Tissue Organ Cult 22: 201–204
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ainsley, P., Aryan, A. Efficient plant regeneration system for immature embryos of triticale (x Triticosecale Wittmack). Plant Growth Regulation 24, 23–30 (1998). https://doi.org/10.1023/A:1005980410397
Issue Date:
DOI: https://doi.org/10.1023/A:1005980410397