Abstract
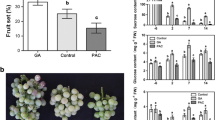
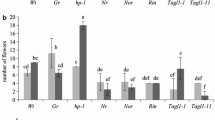
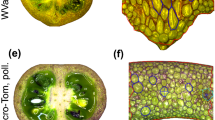
In vitro culture of VFNT Cherry tomato sepals (calyx) at 16–21 °C results in developmental changes that are similar to those that occur in fruit tissue [10]. Sepals become swollen, red, and succulent, produce ethylene, and have increased levels of polygalacturonase RNA. They also produce many flavor volatiles characteristic of ripe tomato fruit and undergo similar changes in sugar content [11]. We examined the expression of the tomato AGAMOUS gene, TAG1, in ripening, in vitro sepal cultures and other tissues from the plant and found that TAG1 RNA accumulates to higher levels than expected from data from other plants. Contrary to reports on the absence of AGAMOUS in sepals, TAG1 RNA levels in green sepals from greenhouse-grown plants is detectable, its concentration increasing with in vitro ripening to levels that were even higher than in red, ripe fruit. Sepals of fruit on transgenic tomato plants that expressed TAG1 ectopically were induced by low temperature to ripen in vivo, producing lycopene and undergoing cell wall softening as is characteristic of pericarpic tissue. We therefore propose that the induction of elevated TAG1 gene expression plays a key role in developmental changes that result in sepal ripening.
Similar content being viewed by others
References
Bowman JL, Drews GN, Meyerowitz EM: Expression of the Arabidopsis floral homeotic gene AGAMOUS is restricted to specific cell types late in flower development. Plant Cell 3: 749–758 (1991).
Bowman JL, Smyth DR, Meyerowitz EM: Genes directing flower development in Arabidopsis. Plant Cell 1: 37–52 (1989).
Bowman JL, Smyth DR, Meyerowitz EM: Genetic interactions among floral homeotic genes of Arabidopsis. Development 112: 1–20 (1991).
Bradley D, Carpenter R, Sommer H, Hartley N, Coen E: Complementary floral homeotic phenotypes result from opposite orientations of a transposon at the plena locus of Antirrhinum. Cell 72: 85–95 (1993).
Crookes PR, Grierson D: Ultrastructure of tomato fruit ripening and the role of polygalacturonase isoenzymes in cell wall degradation. Plant Physiol 72: 1088–1093 (1983).
Davies JN, Hobson GE: The constituents of tomato fruit. The influence of environment, nutrition, and genotype. CRC Crit Rev Food Sci Nutr 15: 205–280 (1981).
Drews GN, Bowman JL, Meyerowitz EM: Negative regulation of the Arabidopsis homeotic gene AGAMOUS by the APETALA2 product. Cell 65: 991–1002 (1991).
Haughn GW, Somerville CR: Genetic control of morphogenesis in Arabidopsis. Devel Genet 9: 73–89 (1988).
Hobson G: Polygalacturonase in normal and abnormal tomato fruit. Biochem J 92: 324–332 (1964).
Ishida BK: Developmental regulation is altered in the calyx during in vitro ovary culture of tomato. Plant Cell 3: 219–223 (1991).
Ishida BK, Baldwin EA, Buttery RG, Chui SH, Ling LC: Flavor volatiles, sugars and color development in ripening in vitrocultured tomato fruit and calyx. Physiol Plant 89: 861–867 (1993).
Jofuku KD, den Boer BG, Van Montagu M, Okamuro JK: Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. Plant Cell 6: 1211–1225 (1994).
Kempin SA, Mandel MA, Yanofsky MF: Ectopic expression of the tobacco floral homeotic gene NAG1 converts perianth into reproductive organs. Plant Physiol 103: 1041–46 (1993).
Komaki MK, Okada K, Nishino E, Shimura Y: Isolation and characterization of novel mutants of Arabidopsis thaliana defective in flower development. Development 104: 195–203 (1988).
Kunst L, Klenz JE, Haughn G: AP2 gene determines the identity of perianth organs in flowers of Arabidopsis thaliana. Plant Cell 1: 1195–1208 (1989).
Mandel MA, Bowman JL, Kempin SA, Ma H, Meyerowitz EM, Yanofsky MF: Manipulation of flower structure in transgenic tobacco. Cell 71: 133–143 (1992).
Mizukami Y, Ma H: Ectopic expression of the floral homeotic gene AGAMOUS in transgenic Arabidopsis plants alters floral organ identity. Cell 71: 119–131 (1992).
Mizukami Y, Ma H: Separation of AG function in floral meristem determinacy from that in reproductive organ identity by expressing antisense AG RNA. Plant Mol Biol 28: 767–784 (1995).
Murashige T, Skoog F: A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 (1962).
Pnueli L, Haraven D, Rounsley SD, Yanofsky MF, Lifschitz E: Isolation of the tomato AGAMOUS gene TAG1 and analysis of its homeotic role in transgenic plants. Plant Cell 6: 163–173 (1994).
Schwarz-Sommer Z, Huijser P, Nacken W, Saedler H, and SommerH:Genetic control of flower development by homeotic genes in Antirrhinum majus. Science 250: 931–936 (1990).
Sieburth LE, Running MP, Meyerowitz EM: Genetic separation of third and fourth whorl functions of AGAMOUS. Plant Cell 7: 1249–1258 (1995).
Tsuchimoto S, van der Krol AR, Chua NH: Ectopic expression of pMADS3 in petunia phenocopies the petunia blind mutant. Plant Cell 5: 843–853 (1993).
White PR: The Cultivation of Animal and Plant Cells, 2nd ed, p. 60. Ronald Press Co, New York (1963).
Yanofsky MF: Floral meristems to floral organs: genes controlling early events in Arabidopsis flower development, Annu Rev Plant Physiol Plant Mol Biol 46: 167–188 (1995).
Yanofsky MF, Ma H, Bowman JL, Drews BN, Feldmann KA, and Meyerowitz EM: The protein encoded by the Arabidopsis homeotic gene AGAMOUS resembles transcription factors. Nature 346: 35–39 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ishida, B.K., Jenkins, S.M. & Say, B. Induction of AGAMOUS gene expression plays a key role in ripening of tomato sepals in vitro. Plant Mol Biol 36, 733–739 (1998). https://doi.org/10.1023/A:1005941330004
Issue Date:
DOI: https://doi.org/10.1023/A:1005941330004