Abstract
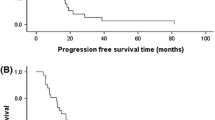
Purpose. Treatment results in patients failing first-line chemotherapy inmetastatic breast cancer (MBC) are still unsatisfactory, with patientsexhibiting poor responses to salvage therapy and a short overall survival.Both paclitaxel and ifosfamide are able to produce objective tumor responsesin the disease. Therefore, the antitumor effects and toxicity of theircombined use could be worthwhile studying in patients progressing afterdoxorubicin-containing combinations. Patients and methods: This Phase IItrial of paclitaxel/ifosfamide included patients with bi-dimensionallymeasurable metastatic breast cancer in second or third relapse, followinganthracycline-containing regimens: ECOG PS <2, and adequate hepatic,cardiac renal and hematological functions. Paclitaxel 175 mg/m2 was given onday 1, in a 3-hour infusion with appropriate antiallergic pre-medication;while ifosfamide 1.8 g/m2 was given on days 2, 3, 4 with mesna 360 mg/m2i.v., 15 minutes before and 4 hours after ifosfamide administration, and 720mg/m2 P.O. 8 hours later at home, also on days 2, 3, 4. The cycles wererepeated every 21 days, on an outpatient basis. Results. Twenty-fourpatients were accrued for the study and 23 were considered eligible for theevaluation of toxicity ans response. Previous chemotherapy included: CMF/FAC(16 cases); CMF plus mitoxantrone/FAC/cisplatin, vinblastine, mitomycin C (2cases); and FAC/mitomycin C, vinblastine, and etoposide (5 cases). Therewere 11 (48%) objective responses (95% C.I.: 27-69%),including 2 (9%) CR and 9 (39%) PR (95%) C.I.:0-21% and 19-61%, respectively). Five (22%) patientsattained disease stabilization. Median response duration was 7+ months(range 4 to 20+), and the median overall survival was 12 months (range4-23+). The regimen was well tolerated. WHO nausea/vomiting grades 1-2,alopecia grade 3, and neutropenia grades 1-2 were seen in most patients.Four patients experienced mild neuropathy, while it was grade 3 in 1 case.Seven patients had grade 3 neutropenia. In addition, grade 4 neutropeniaassociated with fever was documented in other 4 cases. No hypersensitivityreactions were seen. One case of reversible tachycardia after drugadministration was seen. Myalgia grades 1-2 was also reported in somepatients. Conclusion. These results suggest that the present regimen hassignificant activity in heavily pretreated patients with a MBC, with amanageable toxicity profile. Further trials exploiting the above mentioneddrug combination are warranted.
Similar content being viewed by others
References
Henderson CI: Chemotherapy of breast cancer: a general overview. Cancer 51: 2553–2559, 1983
Schwartsmann G, Pinedo HM: Clinical trials in advanced breast cancer. Eur J Cancer 23: 595–597, 1987
Henderson IC, Garber JE, Breitmeyer JB: Comprehensive management of disseminated breast cancer. Cancer 66: 1439–1448, 1990
Kingston DGI, Samaranayake G, Ivey CA: The chemistry of paclitaxel, a clinically useful anticancer agent. J Nat Prod 53: 1–12, 1990
Wani MC, Taylor HL, Wall ME et al.: Plant antitumor agents VI. The isolation and structure of paclitaxel, a novel antileukemic and antitumor agent from taxus brevifolia. J Am Chem Soc 893: 2325–2327, 1971
McGuire WP, Rowinsky EK, Rosenshein NB et al.: Paclitaxel: a unique antineoplastic agent with significant activity in advanced ovarian epithelial neoplasms. Ann Int Med 11: 273–279, 1989
Chang A, Kim K, Glick J et al.: Phase II study of paclitaxel in patients with stage IV nonsmall cell lung cancer. The Eastern Cooperative Study Group study results (abst). Proc Am Soc Clin Oncol 11: 294, 1992
Forastiere AA, Adams G, Neuberg D et al.: Phase II trial of paclitaxel in head and neck cancer: an Eastern Cooperative Study Group study (abst). Second National Cancer Institute Workshop on Paclitaxel and Taxus. Alexandria, VA 1992
Holmes FA, Walters RS, Theriault RL et al.: Phase II trial of paclitaxel, an active drug in the treatment of metastatic breast cancer. J Natl Cancer Inst 83: 1797–1805, 1991
Seidman AD, Tiersten A, Hudis C et al.: Phase II trial of paclitaxel by 3-hour infusion as initial and salvage chemotherapy for metastatic breast cancer. J Clin Oncol 13: 2575–2581, 1995
Reichman BS, Seidman AD, Crown JPA et al.: Paclitaxel and recombinant human granulocyte colony-stimulating factor as initial chemotherapy for metastatic breast cancer. J Clin Oncol 11: 1943–1951, 1993
Holmes FA, Valero V, Walters RS et al.: The M.D. Anderson Cancer Center experience with paclitaxel in metastatic breast cancer. Monogr Natl Cancer Inst 15: 161–169, 1993
Seidman AD, Hudis CA, Norton L: Memorial Sloan-Kettering Cancer Center Experience with paclitaxel in the treatment of breast cancer: from advanced disease to adjuvant therapy. Semin Oncol 22(4 Suppl 8): 3–8, 1995
Hortobagyi GN: Activity of ifosfamide in breast cancer. Semin Oncol 19(6 Suppl 12): 36–41, 1992
Becher R, Hofeler H, Kloke O et al.: Isofamide, methotrexate and 5-FU in advanced pretreated breast cancer. Semin Oncol 16: 56–59, 1989
Fields KK, Effenbein GJ, Perkins JB et al.: Two novel high-dose treatment regimens for metastatic breast cancer — ifosfamide, carboplatin, plus etoposide and mitoxantrone plus thiotepa: outcomes and toxicities. Semin Oncol 20: 59–66, 1993
WHO: Handbook for Reporting Results of Cancer Treatment. WHO Offset publication No 48. World Health Organization, Geneva, Switzerland, 1979
Schwartsmann G, Wanders J, Koier IJ, Franklin HR, Dalesio O, Hornstra HW, van Glabbeke, Renard J, van Oosterom AT, Kaye SB, Pinedo HM: EORTC New Drug Development Office coordinating and monitoring program for phase I and II trails with new anticancer drugs. Eur J Cancer 9: 1162–1168, 1991
Gehan EA: The determination of the number of patients required in a follow up trial of a new chemotherapy agent. J Chronic Dis 13: 346–353, 1961
Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53: 457–481, 1958
Campos-Filho N, Franco ELF: Microcomputer-assisted univariate survival data analysis using Kaplan-Meier life table estimators. Comp Meth Prog Biomed 27: 223–228, 1988
Norton L, Simon R: The Norton-Simon hypothesis revisited. Cancer Treat Rep 70: 163–169, 1986
Gianni L, Munzone E, Capri G et al.: Paclitaxel by 3-hour infusion in combination with bolus doxorubicin in women with untreated metastatic breast cancer. High antitumor efficacy and cardiac effects in a dose-finding and sequence-finding study. J Clin Oncol 13: 2688–2699, 1955
Schwartsmann G, Menke CH, Caleffi M, Xavier N, Ferreira Filho AF, Schunemann H, Koya R, Stroda PR, Pohlmann P, Venegas LF, Kalakun L: Phase II trial of paclitaxel, plus doxorubicin plus G-CSF in patients with metastatic breast cancer. Proc Am Soc Clin Oncol 15: 125, 1996 (abstr)
Klaassen U, Wilke H, Philippou Pari C et al.: Phase I/II study with paclitaxel in combination with weekly high-dose 5-FU/folonic acid in the treatment of metastatic breast cancer. Proc Am Soc Clin Oncol 15 (abstr 186), 1995
Murad AM, Quirino RM, Morici AM et al.: Phase II trial of the use of paclitaxel and ifosfamide in heavily pre-treated patients with metastatic breast cancer. Breast Cancer Res Treat 37: 89, 1996 Abstract (Suppl 1)
Rosenthal CJ, Ibrahim A, Rambhia H et al.: Paclitaxel mitoxantrone salvage therapy in metastatic carcinoma of the breast-Phase I/II study. Proc Am Soc Clin Oncol 13: 92, 1994 (abstr)
Paul DM, Garrett AM, Meshad M et al.: A phase II trial of paclitaxel, 5-fluorouracil and leucovorin (TFL) in metastatic breast cancer. Proc Am Soc Clin Oncol 14: 140, 1995 (abstr)
Murad AM, Tinoco LA, Schwartsmann G, Amorim WC: Phase II trial of the use of paclitaxel and ifosfamide in heavily pre-treated patients with metastatic breast cancer. Proc Am Soc Clin Oncol 15: 97, 1996
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Murad, A., Guimaraes, R., Amorim, W. et al. Phase II trial of paclitaxel and ifosfamide as a salvage treatment in metastatic breast cancer. Breast Cancer Res Treat 45, 47–53 (1997). https://doi.org/10.1023/A:1005882314735
Issue Date:
DOI: https://doi.org/10.1023/A:1005882314735