Abstract
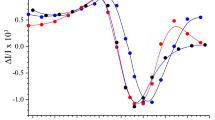
To establish a system for over-production of PSII-L protein which is a component of photosystem II (PSII) complex, a plasmid designated as pMAL-psbL was constructed and expressed in Escherichia coli JM109. A fusion protein of PSII-L and maltose-binding proteins (53 kDa on SDS-PAGE) was accumulated in E. coli cells to a level of 10% of the total protein upon isopropyl-β-D-thiogalactopyranoside (IPTG) induction. The carboxyl-terminal part of 5.0 kDa was cleaved from the fusion protein and purified by an anion exchange column chromatography in the presence of detergents. This 5.0 kDa protein was identified as PSII-L by amino-terminal amino acid sequence analysis and the chromatographic behavior on an anion exchange gel. A few types of mutant PSII-L were also prepared by the essentially same procedure except for using plasmids which contain given mutations in psbL gene. Plastoquinone-9 (PQ-9) depleted PSII reaction center core complex consisting of D1, D2, CP47, cytochrome b-559 (cyt b-559), PSII-I and PSII-W was reconstituted with PQ-9 and digalactosyldiglyceride (DGDG) together with the wild-type or mutant PSII-L produced in E. coli or isolated PSII-L from spinach. Significant difference between the wild-type PSII-L proteins from E. coli and spinach was not recognized in the effectiveness to recover the photo-induced electron transfer activity in the resulting complexes. The analysis of stoichiometry of PQ-9 per reaction center in the PQ-9 reconstituted PS II revealed that two molecules of PQ-9 were reinserted into a reaction center independent of the presence or absence of PSII-L. These results suggest that PSII-L recovers the electron transfer activity in the reconstituted RC by a mechanism different from the stabilization of PQ-9 in the QA site of PSII. Ubiquinone-10 (UQ-10), but not plastoquinone-2 (PQ-2), substituted PQ-9 for recovering the PSII-L supported electron transfer activity in the reconstituted PSII reaction center complexes. The results obtained with the mutant PSII-L proteins revealed that the carboxyl terminal part rather than amino terminal part of PSII-L is crucial for recovering the electron transfer activity in the reconstituted complexes.
Similar content being viewed by others
References
Anbudurai PR, Pakrasi HB: Mutational analysis of the psbLprotein of Photosystem II in the cyanobacterium Synechocystissp. PCC 6803. Z Naturforsch 48c: 267-274 (1993).
Araga C, Akabori K, Sasaki J, Maeda A, Shiina T, Toyoshima Y: Functional reconstitution of the primary quinone acceptor, QA, in the Photosystem II core complex. Biochim Biophys Acta 1142: 36-42 (1993).
Chapman DJ, Gounaris K, Barber J: Electron-transport properties of the isolated D1-D2-cytochrome b-559 photosystem II reaction center. Biochim Biophys Acta 933: 423-431 (1988).
Cushman JC, Christopher DA, Little MC, Hallick RB, Price CA: Organization of the psbE, psbF, orf38, and orf42gene loci on the Euglena gracilischloroplast genome. Curr Genet 13: 173-180 (1988).
Herrmann RG, Alt J, Schiller B, Widger WR, Cramer WA: Nucleotide sequence of the gene for apocytochrome b-559 on the spinach plastid chromosome: implications for the structure of the membrane protein. FEBS Lett 176: 239-244 (1984).
Ikeuchi M, Inoue Y: Anew 4.8-kDa polypeptide intrinsic to the PSII reaction center, as revealed by modified SDS-PAGE with improved resolution of low-molecular-weight proteins. Plant Cell Physiol 29: 1233-1239 (1988).
Ikeuchi M, Takio K, Inoue Y: N-terminal sequencing of photosystem II low-molecular-mass proteins 5 and 4.1 kDa components of O2-evolving core complex from higher plants. FEBS Lett 242: 263-269 (1989).
Kitamura K, Ozawa S, Shiina T, Toyoshima Y: L protein, encoded by psbL, restores normal functioning of the primary quinone acceptor, QA, in isolated D1/D2/CP47/Cytb-559/I photosystem II reaction center core complex. FEBS Lett 354: 113-116 (1994).
Kudla J, Igloi GL, Metzlaff M, Hagemann R, Kössel H: RNA editing in tobacco chloroplasts leads to the formation of a translatable psbLmessenger RNA by a C-to-U substitution within the initiation codon. EMBO J 11: 1099-1103 (1992).
Kunkel TA, Roberts JD, Zakour RA: Rapid and efficient site-specific mutagenesis without phenotypic selection. Meth Enzymol 154: 367-382 (1987).
Maina CV, Riggs PD, Grandea III AG, Slatko BE, Moran LS, Tagliamonte JA, McReynolds LA, Guan C: An Escherichia colivector to express and purify foreign proteins by fusion to and separation from maltose-binding protein. Gene 74: 365- 373 (1988).
Mathis P, Satoh K, Hasson Ö: Kinetics evidence for the function of Z in isolated photosystem II reaction center. FEBS Lett 251: 241-244 (1989).
Miyazaki A, Shiina T, Toyoshima Y, Gounaris K, Barber J: Stoichiometry of cytochrome b-559 in Photosystem II. Biochim Biophys Acta 975: 142-147 (1989).
Moser CC, Keske JM, Warncke K, Farid RS, Dutton PL: Nature of biological electron transfer. Nature 355: 796-802 (1992).
Nagai K, Togersen HC: Synthesis and sequence-specific proteolysis of hybrid proteins produced in Escherichia coli. Meth Enzymol 153: 461-481 (1987).
Nagatsuka T, Fukuhara H, Akabori K, Toyoshima Y: Disintegration and reconstitution of Photosystem II reaction center core complex. II. Possible involvement of low-molecular-mass proteins in the functioning of QA in the PSII reaction center. Biochim Biophys Acta 1057: 223-231 (1991).
Nanba O, Satoh K: Isolation of a photosystem II reaction center consisting of D1 and D2 polypeptides and cytochrome b559. Proc Natl Acad Sci USA 84: 109-112 (1987).
Satoh K, Hasson Ö, Mathis P: Charge recombination between stabilized P-680+ and reduced cytochrome b559 in quinonereconstituted PSII reaction center. Biochim Biophys Acta 1061: 121-126 (1990).
Shinozaki K, Ohme M, Tanaka M, Wakasugi T, Hayashida N, Matsubayashi T, Zaita N, Chunwongse J, Obokata J, Yamaguchi-Shinozaki K, Ohto C, Torazawa K, Meng BY, Sugita M, Deno H, Kamogashira T, Yamada K, Kusuda J, Takaiwa F, Kato A, Tohdoh N, Shimada H, Sugiura M: The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J 5: 2043-2049 (1986).
Toyoshima Y, Fukutaka E: A protein essential for recovering oxygen evolution in cholate-treated chloroplast. FEBS Lett 150: 223-227 (1982).
Weber AN, Hird SM, Packman LC, Dyer TA, Gray JC: A photosystem II polypeptide is encoded by an open reading frame co-transcribed with genes for cytochrome b-599 in wheat chloroplast DNA. Plant Mol Biol 12: 141-151 (1989).
Woodbury NW, Parson WW, Gunner MR, Prince RC, Dutton PL: Radical-pair energetics and decay mechanisms in reaction center containing anthraquinones, naphtoquinones or benzoquinones in place of ubiquinone. Biochim Biophys Acta 851: 6-22 (1986).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ozawa, S., Kobayashi, T., Sugiyama, R. et al. Role of PSII-L protein (psbL gene product) on the electron transfer in photosystem II complex. 1. Over-production of wild-type and mutant versions of PSII-L protein and reconstitution into the PSII core complex. Plant Mol Biol 34, 151–161 (1997). https://doi.org/10.1023/A:1005800909495
Issue Date:
DOI: https://doi.org/10.1023/A:1005800909495