Abstract
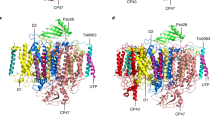
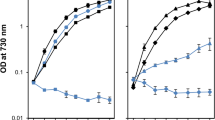
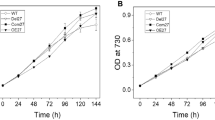
Photosystem II (PSII), the oxygen-evolving enzyme, consists of 17 trans-membrane and 3 extrinsic membrane proteins. Other subunits bind to PSII during assembly, like Psb27, Psb28, and Tsl0063. The presence of Psb27 has been proposed (Zabret et al. in Nat Plants 7:524–538, 2021; Huang et al. Proc Natl Acad Sci USA 118:e2018053118, 2021; Xiao et al. in Nat Plants 7:1132–1142, 2021) to prevent the binding of PsbJ, a single transmembrane α-helix close to the quinone QB binding site. Consequently, a PSII rid of Psb27, Psb28, and Tsl0034 prior to the binding of PsbJ would logically correspond to an assembly intermediate. The present work describes experiments aiming at further characterizing such a ∆PsbJ–PSII, purified from the thermophilic Thermosynechococcus elongatus, by means of MALDI-TOF spectroscopy, thermoluminescence, EPR spectroscopy, and UV–visible time-resolved spectroscopy. In the purified ∆PsbJ–PSII, an active Mn4CaO5 cluster is present in 60–70% of the centers. In these centers, although the forward electron transfer seems not affected, the Em of the QB/QB− couple increases by ≥ 120 mV , thus disfavoring the electron coming back on QA. The increase of the energy gap between QA/QA− and QB/QB− could contribute in a protection against the charge recombination between the donor side and QB−, identified at the origin of photoinhibition under low light (Keren et al. in Proc Natl Acad Sci USA 94:1579–1584, 1997), and possibly during the slow photoactivation process.








Similar content being viewed by others
Abbreviations
- Chl:
-
Chlorophyll
- ChlD1/ChlD2 :
-
Accessory Chl’s on the D1 or D2 side, respectively
- DCMU:
-
3-(3,4-Dichlorophenyl)-1,1-dimethylurea
- PSII:
-
Photosystem II
- MES:
-
2–(N–Morpholino) ethanesulfonic acid
- P680 :
-
Primary electron donor
- PD1 and PD2 :
-
Chl monomer of P680 on the D1 or D2 side, respectively, PheD1 and PheD2, pheophytin on the D1 or D2 side, respectively
- Q A :
-
Primary quinone acceptor
- Q B :
-
Secondary quinone acceptor
- TyrD :
-
Redox active tyrosine 160 of D2
- TyrZ :
-
Redox active tyrosine 161 of D1
- TL:
-
Thermoluminescence
- WT*3:
-
T. elongatus Mutant strain containing only the psbA3 gene and a His6-tag on the C-terminus of CP43
- EPR:
-
Electron paramagnetic resonance spectroscopy
- MALDI-TOF:
-
Matrix-assisted laser desorption ionization-time of flight
References
Bao H, Burnap RL (2016) Photoactivation: the light-driven assembly of the water oxidation complex of Photosystem II. Front Plant Sci. https://doi.org/10.3389/fpls.2016.00578
Béal D, Rappaport F, Joliot P (1999) A new high-sensitivity 10-ns time-resolution spectrophotometric technique adapted to in vivo analysis of the photosynthetic apparatus. Rev Sci Instrum 70:202–207. https://doi.org/10.1063/1.1149566
Boussac A, Sugiura M, Rappaport F (2011) Probing the quinone binding site of photosystem II from Thermosynechococcus elongatus containing either PsbA1 or PsbA3 as the D1 protein through the binding characteristics of herbicides. Biochim Biophys Acta 1807:119–129. https://doi.org/10.1016/j.bbabio.2010.10.004
Broser M, Gabdulkhakov A, Kern J, Guskov A, Muh F, Saenger W, Zouni A (2010) Crystal structure of monomeric Photosystem II from Thermosynechococcus elongatus at 3.6-A˚ resolution. J Biol Chem 285:26255–26262. https://doi.org/10.1074/jbc.M110.127589
Choo P, Forsman JA, Hui LL, Khaing EP, Summerfield TC, Eaton-Rye JJ (2021) The PsbJ protein is required for photosystem II activity in centers lacking the PsbO and PsbV lumenal subunits. Photosynth Res. https://doi.org/10.1007/s11120-021-00862-y
Cox N, Pantazis DA, Lubitz W (2020) Current understanding of the mechanism of water oxidation in Photosystem II and its relation to XFEL data. Annu Rev Biochem 89:795–820. https://doi.org/10.1146/annurev-biochem-011520-104801
Cser K, Vass I (2007) Radiative and non-radiative charge recombination pathways in Photosystem II studied by thermoluminescence and chlorophyll fluorescence in the cyanobacterium Synechocystis 6803. Biochim Biophys Acta 1767:233–243. https://doi.org/10.1016/j.bbabio.2007.01.022
Cuni A, Xiong L, Sayre R, Rappaport F, Lavergne J (2004) Modification of the pheophytin midpoint potential in Photosystem II: modulation of the quantum yield of charge separation and of charge recombination pathways. Phys Chem Chem Phys 6:4825–4831. https://doi.org/10.1039/B407511K
de Causmaecker S, Douglass JS, Fantuzzi A, Nitschke W, Rutherford AW (2019) Energetics of the exchangeable quinone, QB, in Photosystem II. Proc Natl Acad Sci USA 116:19458–19463. https://doi.org/10.1073/pnas.1910675116
Demeter S, Goussias C, Bern G, Kovács L, Petrouleas V (1993) Participation of the g = 1.9 and g = 1.82 EPR forms of the semiquinone-iron complex, QA-Fe2+ of photosystem II in the generation of the Q and C thermoluminescence bands, respectively. FEBS 336:352–356. https://doi.org/10.1016/0014-5793(93)80836-J
Diner BA, Petrouleas V (1987) Light-induced oxidation of the acceptor-side Fe(ll) of Photosystem II by exogenous quinones acting through the QB binding site. II. Blockage by inhibitors and their effects on the FeIII) EPR spectra. Biochim Biophys Acta 893:138–148. https://doi.org/10.1016/0005-2728(87)90033-8
Ducruet JM (2003) Chlorophyll thermoluminescence of leaf discs: simple instruments and progress in signal interpretation open the way to new ecophysiological indicators. J Exp Bot 54:2419–2430. https://doi.org/10.1093/jxb/erg268
Ducruet JM, Vass I (2009) Thermoluminescence: experimental. Photosynth Res 201:195–204. https://doi.org/10.1007/s11120-009-9436-0
Forsman JA, Eaton-Rye JJ (2021) The interaction between PsbT and the DE loop of D1 in Photosystem II stabilizes the quinone-iron electron acceptor complex. Biochemistry 60:53–63. https://doi.org/10.1021/acs.biochem.0c00668
Fufezan C, Zhang CX, Krieger-Liszkay A, Rutherford AW (2005) Secondary quinone in photosystem II of Thermosynechococcus elongatus: semiquinone-iron EPR signals and temperature dependence of electron transfer. Biochemistry 44:12780–12789. https://doi.org/10.1021/bi051000k
Funk C (2000) Functional analysis of the PsbX protein by deletion of the corresponding gene in Synechocystis sp. PCC 6803. Plant Mol Biol 44:815–827. https://doi.org/10.1023/A:1026764728846
Garcia-Cerdan JG, Sveshnikov D, Dewez D, Jansson S, Funk C, Schroder WP (2009) Antisense inhibition of the PsbX protein affects PSII integrity in the higher plant Arabidopsis thaliana. Plant Cell Physiol 50:191–202. https://doi.org/10.1093/pcp/pcn188
Holzwarth AR, Müller MG, Reus M, Nowaczyk M, Sander J, Rögner M (2006) Kinetics and mechanism of electron transfer in intact photosystem II and in the isolated reaction center: pheophytin is the primary electron acceptor. Proc Natl Acad Sci USA 103:6895–6900. https://doi.org/10.1073/pnas.0505371103
Huang G, Xiao Y, Pi X, Zhaoa L, Zhu Q, Wang W, Kuang T, Han G, Sui S-F, Shen J-R (2021) Structural insights into a dimeric Psb27-photosystem II complex from a cyanobacterium Thermosynechococcus vulcanus. Proc Natl Acad Sci USA 118:e2018053118. https://doi.org/10.1073/pnas.2018053118
Hughes JL, Cox N, Rutherford AW, Krausz E, Lai T-L, Boussac A, Sugiura M (2010) D1 protein variants in Photosystem II from Thermosynechococcus elongatus studied by low temperature optical spectroscopy. Biochim Biophys Acta 1797:11–19. https://doi.org/10.1016/j.bbabio.2009.07.007
Inoue-Kashino N, Kashino Y, Takahashi Y (2011) Psb30 is a photosystem II reaction center subunit and is required for optimal growth in high light in Chlamydomonas reinhardtii. J Photochem Photobiol 104:220–228. https://doi.org/10.1016/j.jphotobiol.2011.01.024
Ishida N, Sugiura M, Rappaport F, Lai T-L, Rutherford AW, Boussac A (2008) Biosynthetic exchange of bromide for chloride and strontium for calcium in the photosystem II oxygen-evolving enzyme. J Biol Chem 283:13330–13340. https://doi.org/10.1074/jbc.M710583200
Iwai M, Suzuki T, Kamiyama A, Sakurai I, Dohmae N, Inoue Y, Ikeuchi M (2010) The PsbK subunit is required for the stable assembly and stability of other small subunits in the PSII complex in the thermophilic cyanobacterium Thermosynechococcus elongatus BP-1. Plant Cell Physiol 51:554–560. https://doi.org/10.1093/pcp/pcq020
Johnson GN, Rutherford AW, Krieger A (1995) A change in the midpoint potential of the quinone QA in Photosystem II associated with photoactivation of oxygen evolution. Biochim Biophys Acta 1229:202–207. https://doi.org/10.1016/0005-2728(95)00003-2
Joliot P, Kok B (1975) Oxygen evolution in photosynthesis. In: Govindjee (ed) Bioenergetics of photosynthesis. Academic Press, New York, pp 387–412.
Kaminskaya O, Shuvalov VA, Renger G (2007) Evidence for a novel quinone-binding site in the photosystem II (PS II) complex that regulates the redox potential of cytochrome b559. Biochemistry 46:1091–1105. https://doi.org/10.1134/S1607672907010048
Kashino Y, Lauber WM, Carroll JA, Wang Q, Whitmarsh J, Satoh K, Pakrasi HB (2002) Proteomic analysis of a highly active Photosystem II preparation from the cyanobacterium Synechocystis sp. PCC 6803 reveals the presence of novel polypeptides. Biochemistry 41: 8004–8012. https://doi.org/10.1021/bi026012
Kashino Y, Takahashi T, Inoue-Kashino N, Ban A, Ikeda Y, Satoh K, Sugiura M (2007) Ycf12 is a core subunit in the photosystem II complex. Biochim Biophys Acta 1767:1269–1275. https://doi.org/10.1016/j.bbabio.2007.08.008
Keren N, Berg A, VanKan PJM, Levanon H, Ohad I (1997) Mechanism of photosystem II photoinactivation and D1 protein degradation at low light: The role of back electron flow. Proc Natl Acad Sci USA 94:1579–1584. https://doi.org/10.1073/pnas.94.4.1579
Kok B, Forbush B, McGloin M (1970) Cooperation of charges in photosynthetic O2 evolution–I. A linear four step mechanism. Photochem Photobiol 11:457–475. https://doi.org/10.1111/j.1751-1097.1970.tb06017.x
Komenda J, Sobotka R, Nixon PJ (2012) Assembling and maintaining the Photosystem II complex in chloroplasts and cyanobacteria. Curr Opin Plant Biol 15:245–251. https://doi.org/10.1016/j.pbi.2012.01.017
Krieger A, Rutherford AW, Johnson GN (1995) On the determination of redox midpoint potential of the primary quinone electron acceptor, QA, in photosystem II. Biochim Biophys Acta 1229:193–201. https://doi.org/10.1016/0005-2728(95)00002-Z
Lavergne J (1991) Improved UV-visible spectra of the S-transitions in the photosynthetic oxygen-evolving system. Biochim Biophys Acta 1060:175–188. https://doi.org/10.1016/S0005-2728(09)91005-2
Liu HJ, Chen JW, Huang RYC, Weisz D, Gross ML, Pakrasi HB (2013) Mass spectrometry-based footprinting reveals structural dynamics of loop e of the chlorophyll-binding protein CP43 during Photosystem II assembly in the Cyanobacterium Synechocystis 6803. J Biol Chem 288:14212–14220. https://doi.org/10.1074/jbc.M113.467613
Luo H, Jackson SA, Fagerlund RD, Summerfield TC, Eaton-Rye JJ (2014) The importance of the hydrophilic region of PsbL for the plastoquinone electron acceptor complex of Photosystem II. Biochim Biophys Acta 1837:1435–1446. https://doi.org/10.1016/j.bbabio.2014.02.015
Merry SAP, Nixon PJ, Barter LMC, Schilstra MJ, Porter G, Barber J, Durrant JR, Klug D (1998) Modulation of quantum yield of primary radical pair formation in photosystem II by site directed mutagenesis affecting radical cations and anions. Biochemistry 37:17439–17447. https://doi.org/10.1021/bi980502d
Müh F, Zouni A (2005) Extinction coefficients and critical solubilisation concentrations of photosystems I and II from Thermosynechococcus elongatus. Biochim Biophys Acta 1708:219–228. https://doi.org/10.1016/j.bbabio.2005.03.005
Müh F, Glöckner C, Hellmich J, Zouni A (2012) Light-induced quinone reduction in photosystem II. Biochim Biophys Acta 1817:44–65. https://doi.org/10.1016/j.bbabio.2011.05.021
Mulo P, Sicora C, Aro E-M (2009) Cyanobacterial psbA gene family: optimization of oxygenic photosynthesis. Cell Mol Life Sci 66:3697–3710. https://doi.org/10.1007/s00018-009-0103-6
Nowaczyk MM, Hebeler R, Schlodder E, Meyer HE, Warscheid B, Rogner M (2006) Psb27, a cyanobacterial lipoprotein, is involved in the repair cycle of photosystem II. Plant Cell 18:3121–3131. https://doi.org/10.1105/tpc.106.042671
Nowaczyk MM, Krause K, Mieseler M, Sczibilanski A, Ikeuchi M, Rögner M (2012) Deletion of psbJ leads to accumulation of Psb27–Psb28 photosystem II complexes in Thermosynechococcus elongatus. Biochim Biophys Acta 1817:1339–1345. https://doi.org/10.1016/j.bbabio.2012.02.017
Ohad I, Dal Bosco C, Herrmann RG, Meurer J (2004) Photosystem II proteins PsbL and PsbJ regulate electron flow to the plastoquinone pool. Biochemistry 43:2297–2308. https://doi.org/10.1021/bi0348260
Rappaport F, Lavergne J (2009) Thermoluminescence: theory. Photosynth Res 101:205–216. https://doi.org/10.1007/s11120-009-9437-z
Regel RE, Ivleva NB, Zer H, Meurer J, Shestakov SV, Herrmann RG, Pakrasi HB, Ohad I (2001) Deregulation of electron flow within Photosystem II in the absence of the PsbJ protein. J Biol Chem 276:41473–41478. https://doi.org/10.1074/jbc.M102007200
Romero E, Novoderezhkin VI, van Grondelle R (2017) Quantum design of photosynthesis for bio-inspired solar-energy conversion. Nature 543:355–365. https://doi.org/10.1038/nature22012
Roncel M, Boussac A, Zurita JL, Bottin H, Sugiura M, Kirilovsky D, Ortega J-M (2003) Redox properties of the photosystem II cytochromes b559 and c550 in the cyanobacterium Thermosynechococcus elongatus. J Biol Inorg Chem 8:206–216. https://doi.org/10.1007/s00775-002-0406-7
Roose JL, Pakrasi HB (2008) The Psb27 protein facilitates manganese cluster assembly in photosystem II. J Biol Chem 283:4044–4050. https://doi.org/10.1074/jbc.M708960200
Roose JL, Frankel LK, Mummadisetti MP, Bricker TM (2016) The extrinsic proteins of photosystem II: update. Planta 243:889–908. https://doi.org/10.1007/s00425-015-2462-6
Rutherford AW, Krieger-Liszkay A (2001) Herbicide-induced oxidative stress in photosystem II. Trends Biochem Sci 26:648–653. https://doi.org/10.1016/S0968-0004(01)01953-3
Rutherford AW, Zimmermann J-L (1984) A new EPR signal attributed to the primary plastosemiquinone acceptor in Photosystem II. Biochim Biophys Acta 767:168–175. https://doi.org/10.1016/0005-2728(84)90092-6
Rutherford AW, Crofts AR, Inoue Y (1982) Thermoluminescence as a probe of Photosystem II photochemistry. The origin of the flash-induced glow peaks. Biochim Biophys Acta 682:457–465. https://doi.org/10.1016/0005-2728(82)90061-5
Rutherford AW, Zimmermann J-L, Mathis P (1983) The effect of herbicides on components of the PS II reaction centre measured by EPR. Febs Lett 165:156–162. https://doi.org/10.1016/0014-5793(84)80161-1
Sedoud A, Cox N, Sugiura M, Lubitz W, Boussac A, Rutherford AW (2011) The semiquinone-iron complex of Photosystem II: EPR signals assigned to the low field edge of the ground state doublet of QA·-Fe2+ and QB·-Fe2+. Biochemistry 50:6012–6021. https://doi.org/10.1021/bi200313p
Schatz GH, van Gorkom HJ (1985) Absorbance difference spectra upon charge transfer to secondary donors and acceptors in Photosystem II. Biochim Biophys Acta 810:283–294. https://doi.org/10.1016/0005-2728(85)90212-9
Shen JR (2015) The structure of Photosystem II and the mechanism of water oxidation in photosynthesis. Annu Rev Plant Biol 66:23–48. https://doi.org/10.1146/annurev-arplant-050312-120129
Shen J-R, Qian M, Inoue Y, Burnap RL (1998) Functional characterization of Synechocystis sp. PCC 6803 ∆psbU and ∆psbV mutants reveals important roles of cytochrome c-550 in cyanobacterial oxygen evolution. Biochemistry 37:1551–1558. https://doi.org/10.1021/bi971676i
Sheridan KJ, Duncan EJ, Eaton-Rye JJ, Summerfield TC (2020) The diversity and distribution of D1 proteins in cyanobacteria. Photosynth Res 145:111–128. https://doi.org/10.1007/s11120-020-00762-7
Shibuya Y, Takahashi R, Okubo T, Suzuki H, Sugiura M, Noguchi T (2010) Hydrogen bond interaction of the pheophytin electron acceptor and its radical anion in Photosystem II as revealed by Fourier Transform Infrared Difference Spectroscopy. Biochemistry 49:493–501. https://doi.org/10.1021/bi9018829
Styring S, Rutherford AW (1987) In the oxygen-evolving complex of photosystem II the S0-state is oxidized to the S1-state by D+ (signal-II slow). Biochemistry 26:2401–2405. https://doi.org/10.1021/bi00383a001
Styring S, Rutherford AW (1988) The microwave power saturation of SIIslow varies with the redox state of the oxygen-evolving complex in photosystem II. Biochemistry 27:4915–4923. https://doi.org/10.1021/bi00413a049
Suga M, Akita F, Hirata K, Ueno G, Murakami H, Nakajima Y, Shimizu T, Yamashita K, Yamamoto M, Ago H, Shen J-R (2015) Native structure of photosystem II at 1.95 angstrom resolution viewed by femtosecond X-ray pulses. Nature 517:99–103. https://doi.org/10.1038/nature13991
Sugiura M, Boussac A (2014) Some Photosystem II properties depending on the D1 protein variants in Thermosynechococcus elongatus. Biochim Biophys Acta 1837:1427–1434. https://doi.org/10.1016/j.bbabio.2013.12.011
Sugiura M, Inoue Y (1999) Highly purified thermo-stable oxygen evolving Photosystem II core complex from the thermophilic cyanobacterium Synechococcus elongatus having His-tagged CP43. Plant Cell Physiol 40:1219–1231. https://doi.org/10.1093/oxfordjournals.pcp.a029510
Sugiura M, Rappaport F, Brettel K, Noguchi T, Rutherford AW, Boussac A (2004) Site-directed mutagenesis of Thermosynechococcus elongatus photosystem II: the O2 evolving enzyme lacking the redox active tyrosine D. Biochemistry 43:13549–13563. https://doi.org/10.1021/bi048732h
Sugiura M, Harada S, Manabe T, Hayashi H, Kashino Y, Boussac A (2010a) Psb30 contributes to structurally stabilise the Photosystem II complex in the thermophilic cyanobacterium Thermosynechococcus elongatus. Biochim Biophys Acta 1797:1546–1554. https://doi.org/10.1016/j.bbabio.2010.03.020
Sugiura M, Iwai E, Hayashi H, Boussac A (2010b) Differences in the interactions between the subunits of Photosystem II dependent on D1 protein variants in the thermophilic cyanobacterium Thermosynechococcus elongatus. J Biol Chem 285:30008–30018. https://doi.org/10.1074/jbc.M110.136945
Sugiura M, Azami C, Koyama K, Rutherford AW, Rappaport F, Boussac A (2014) Modification of the pheophytin redox potential in Thermosynechococcus elongatus Photosystem II with PsbA3 as D1. Biochim Biophys Acta 1837:139–148. https://doi.org/10.1016/j.bbabio.2013.09.009
Sugiura M, Taniguchi T, Tango N, Nakamura M, Sellés J, Boussac A (2020) Probing the role of arginine 323 of the D1 protein in photosystem II function. Physiol Plant 171:183–199. https://doi.org/10.1111/ppl.13115
Suhai T, Dencher NA, Poetsch A, Seelert H (2008) Remarkable stability of the proton translocating F1F0-ATP synthase from the thermophilic cyanobacterium Thermosynechococcus elongatus BP-1. Biochim Biophys Acta 1778:1131–1140. https://doi.org/10.1016/j.bbamem.2007.12.017
Takasaka K, Iwai M, Umena Y, Kawakami K, Ohmori Y, Ikeuchi M, Takahashi Y, Kamiya N, Shen JR (2010) Deletion Structural and functional studies on Ycf12 (Psb30) and PsbZ-deletion mutants from a thermophilic cyanobacterium. Biochim Biophys Acta 1797:278–284. https://doi.org/10.1016/j.bbabio.2009.11.001
Umena Y, Kawakami K, Shen J-R, Kamiya N (2011) Crystal structure of oxygen-evolving Photosystem II at a resolution of 1.9 angstrom. Nature 473:55–60. https://doi.org/10.1038/nature09913
Uto S, Kawakami K, Umena Y, Iwai M, Ikeuchi M, Shen JR, Kamiya N (2017) Mutual relationships between structural and functional changes in a PsbM-deletion mutant of photosystem II. Faraday Discuss 198:107–120. https://doi.org/10.1039/C6FD00213G
van Eerden FJ, Melo MN, Frederix PWJM, Periole X, Marrink SJ (2017) Exchange pathways of plastoquinone and plastoquinol in the photosystem II complex. Nat Commun 8:15214. https://doi.org/10.1038/ncomms15214
Velthuys BR (1981) Electron-dependent competition between plastoquinone and inhibitors for binding to Photosystem-II. Feb Let 126:277–281. https://doi.org/10.1016/0014-5793(81)80260-8
Xiao Y, Huang G, You X, Zhu Q, Wang W, Kuang T, Han G, Sui S-F, Shen J-R (2021) Structural insights into cyanobacterial Photosystem II intermediates associated with Psb28 and Tsl0063. Nat Plants 7:1132–1142. https://doi.org/10.1038/s41477-021-00961-7
Zabret J, Bohn S, Schuller SK, Arnolds O, Möller M, Meier-Credo J, Liauw P, Chan A, Tajkhorshid E, Langer JD, Stoll R, Krieger-Liszkay A, Engel BD, Rudack T, Schuller JM, Nowaczyk MM (2021) Structural insights into Photosystem II assembly. Nat Plants 7:524–538. https://doi.org/10.1038/s41477-021-00895-0
Acknowledgements
This work has been in part supported by (i) the French Infrastructure for Integrated Structural Biology (FRISBI) ANR-10-INBS-05, (ii) the Labex Dynamo (ANR-11-LABX-0011-01), (iii) EQUIPEX (CACSICE ANR-11-EQPX-0008), notably through funding of the Proteomic Platform of IBPC (PPI). MS was supported by the JSPS-KAKENHI Grant in Scientific Research on Innovative Areas JP17H06435 and a JSPS-KAKENHI Grant 21H02447.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Boussac, A., Sellés, J., Hamon, M. et al. Properties of Photosystem II lacking the PsbJ subunit. Photosynth Res 152, 347–361 (2022). https://doi.org/10.1007/s11120-021-00880-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-021-00880-w