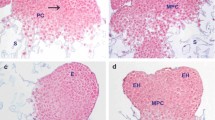
Abstract
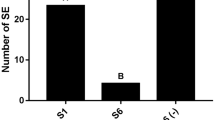
The effect of pretreatments with 0.5, 1, 3, 5 and 10 mM of DL-α-difluoromethylarginine (DFMA), an irreversible suicide inhibitor of the arginine decarboxylase (ADC) activity, on the differentiation process of young maize calluses was studied. Callus protein, total polyamine content and ADC activity decreased versus control in all the assayed treatments. Furthermore, ornithine decarboxylase (ODC) activity was significantly lower in the treated calluses, which was probably due to the arginase activity detected in them. Short tratments at high doses of DFMA significantly increased the number of regenerated buds versus the control (four times more in 10 mM and almost two times more in 5 mM). By contrast, long treatments at low doses (0.5, 1 and 3 mM) reduced the number of plantlets. Conjugated putrescine seems to be implicated in the regeneration response of control and high DFMA-treated calluses.
Similar content being viewed by others
References
Adiga RC and Prasal GL (1985) Biosynthesis and regulation of polyamines in higher plants. Plant Growth Reg. 3: 205–226
Altman A, Friedman K and Levin N (1983) Alternative metabolic pathways for polyamine biosynthesis in plant development. In: Bachrach et al. (eds) Advances in Polyamine Research, vol 4, pp 395–408. New York: Raven Press
Bagni N, Serafini-Fracassini D and Torrigiani P (1982) Polyamines and cellular growth processes in higher plants. In: Wareing PF (ed) Plant Growth Substances, pp 473–492. New York: Academic Press
Bell E and Malmberg RL (1990) Molecular cloning of cDNA encoding arginine decarboxylase from oats. In: Flores HE, Arteca RN and Shannon JC (eds) Polyamines and Ethylene. Biochemistry, Physiology and Interactions, pp 1–23. Proc. 5th Ann Penn State Symp. in Plant Physiol
Bradford MM (1976) A rapid method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254
Burtin D, Martin-Tanguy J, Painot M and Rossi NN (1989) Effects of the suicide inhibitors of arginine and ornithine decarboxylase activities on organogenesis, growth, free polyamine and hydroxycinamoyl putrescine levels in leaf explants of Nicotiana xanti n.c. cultivated in vitro in a medium producing callus formation. Plant Physiol. 89: 104–110
Davtyan MA and Vardanyan DA (1974) Proteolytic and aminase activity in corn grains. Biol. Zh. Arm. 29(12): 3–7
Claparols I, Santos MA and Torné JM (1993) Influence of some exogenous amino acids on the production of maize embryogenic callus and on endogenous amino acid content. Plant Cell, Tissue and Org. Cult. 34: 1–11
Del Duca S and Serafini-Fracasini D (1993) Polyamines and protein modification during the cell cycle. In: Ormrod JC and Francis D (eds) Molecular and Cell Biology of the Plant Cell Cycle, pp 143–156. Netherlands: Kluwer Academic Publishers
Di Tomaso J, Shaff JE and Kochian LV (1989) Putrescine-induced wounding and its effects on membrane integrity and ion transport processes in roots of intact corn seedlings. Plant Physiol. 90: 988–995
Evans PT and Malmberg RL (1989) Do polyamines have roles in plant development? Ann. Rev. Plant Physiol. Plant Mol. Biol. 40: 235–269
Feirer RP, Mignon G and Litway JD (1984) Arginine decarboxylase and polyamines required for embryogenesis in the wild carrot. Science 223: 1433–1435
Galston AW and Kaur-Sawhney R (1990) Polyamines in plant physiology. Plant Physiol. 94: 406–410
Hiatt AC, McIndoo J and Malmberg RL (1986) Regulation of polyamine biosynthesis in tobacco: Effect of inhibitors and exogenous polyamines on arginine decarboxylase, ornithine decarboxylase and S-adenosylmethionine decarboxylase. J. Biochem. 1293–1298
Hiatt A (1989) Polyamine synthesis in maize cell lines. Plant Physiol. 90: 1378–1381
Kaur-Sawhney R, Shahawat NG and Galston AW (1985) Polyamine levels as related to growth, differentiation and senescence in protoplast-derived cultures of Vigna aconitifolia and Avena sativa. Plant Growth Regul. 3: 329–337
Malmberg RL, McIndoo J, Hiatt AC and Lowe BA (1985) Genetics of polyamine synthesis in tobacco development switches in the flower. Cold Spring Harbor Symp. 50: 475–482
Morawska G, Kleckowski K and Reifer I (1963) The occurrence and activity of arginase in higher plants. Acta Bot. Polon. 32: 191–199
Mukhopadhyay A and Ghosh B (1986) Polyamine metabolism in relation to viability of rice seeds. Seed Sci. Technol. 14(3): 737–51
Murashige T and Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15: 473–497
Muszynska G and Reifer I (1970) The arginase inhibitor from sunflower seeds: Purification and inhibitory properties. Acta Biochem. Polon. 17: 247–252
Roubelakis KA and Kliewer WM (1978) Enzymes of Krebs-Henseleit cycle in Vitis vinifera. III. In vivo and in vitro studies of arginase. Plant Physiol. 62: 344–347
Santos MA, Torné JM and Blanco JL (1984) Methods of obtaining maize totipotent tissues: I.-Seedling segments culture. Plant Sci. Lett. 33: 309–315
Santos M, Claparols I and Torné JM (1993) Effect of exogenous arginine, ornithine, methionine and γ-amino butyric acid on maize (Zea mays L.) embryogenesis and polyamine content. J. Plant Physiol. 142(1): 74–80
Santos M, Boget N and Torné JM (1995) Endogenous polyamine content during in vivo maturation and in vitro culture of maize pollen. Plant Growth Reg. 16: 19–25
Shen HJ and Galston AW (1985) Correlation between polyamine ratios and growth patterns in seedling roots. Plant Growth Reg. 3: 353–363
Slocum RD, Kaur-Shawney R and Galston AW (1984) The physiology and biochemistry of polyamines in plants. Arch. Biochem. Biophys. 235: 283–303
Slocum RD, Bionti AJ, McCann PP and Feirer RP (1988) DL-α-difluoromethylarginine metabolism in tobacco and mammalian cells: Inhibition of ornithine decarboxylase activity after arginase-mediates hydrolysis of DL-α-difluoromethylarginine to DL-α-difluoromethylornithine. Biochem. J. 255: 197–202
Smith TA (1990) Plant Polyamines-metabolism and function. In: Flores HE, Arteca RN and Shannon JC (eds) Polyamines and Ethylene: Biochemistry, Physiology and Interactions, pp 1–23. Proc. 5th Ann. Penn State Symp. in Plant Physiol.
Tiburcio AF, Kaur-Sawhney R, Ingersoll RB and Galston AW (1985) Correlation between polyamines and pyrrolidine alkaloids in developing tobacco callus. Plant Physiol. 78: 323–326
Tiburcio A, Kaur-Sawhney R and Galston AW (1988) Polyamine biosynthesis during vegetative and floral bud differentiation in thin-layer tobacco tissue cultures. Plant Cell Physiol. 29:1241–1249
Tiburcio, A., Figueras, X., Claparols, I., Santos, M. and Torné, J.M. (1991) Improved plant regeneration in maize callus cultures after pretreatment with DL-α-difluoromethylarginine. Plant Cell Tissue and Org. Cult. 27: 27–32
Tiburcio AF, Besford RT, Capell T, Borrell A, Testillano PS and Risueño MC (1994) Mechanisms of polyamine action during senescence response induced by osmotic stress. J. Exp. Bot. 45(281): 1789–1800
Torné JM, Santos MA and Blanco JL (1984) Methods of obtaining maize totipotent tissues: II Atrophic tissue culture. Plant Sci. Lett. 33: 317–325
Torné JM and Santos MA (1990) The meristematic calli of maize: A maintenance system of tissue juvenility. In: Rodriguez R, Tamés RS and Duzzan DJ (eds) Plant Aging: Basic and Applied Approaches, pp 395–398. New York: Plenum Press
Torné JM, Claparols I, Marcé M, Guergué A and Santos M (1994) Influence of pretreatments with inhibitors of putrescine synthesis on polyamine metabolism and differentiation processes of maize calluses Plant Sci. 100: 15–22
Torrigiani P, Serafini-Fracassini D, Biondi S and Bagni N (1986) Evidence for the subcellular localization of polyamines and their biosynthetic enzymes in plant cells. J. Plant Physiol. 124: 23–29
Wuensch A and Amberger A (1974) Occurrence of arginine in the metabolism of plants fed with cynamide. Z. Pfalnzenphysiol. 72(3): 359–366
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Guergué, A., Claparols, I., Santos, M. et al. Modulator effect of DL-α-difluoromethylarginine treatments on differentiation processes of young maize calluses. Plant Growth Regulation 21, 7–14 (1997). https://doi.org/10.1023/A:1005785011610
Issue Date:
DOI: https://doi.org/10.1023/A:1005785011610