Abstract
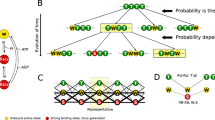
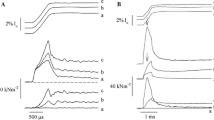
Predictions for the time courses of cross-bridge attachment, N(t), stiffness, S(t), and force, T(t), during the tetanus rise were analysed for a special class of cross-bridge models where cross-bridges initially attach in a non-stereospecific weak-binding state, A w. This state is in rapid equilibrium (equilibrium constant K) with detached states and the force generating transition (rate constant F +) is delayed. One model (model IA) which assumed step-function rise of activation at onset of tetanus, gave a poor fit to the experimental data (judged by root mean square error, RMSe ≈ 0.038) but the experimentally observed lead of N(t) over T(t) was reproduced qualitatively. An activation mechanism where K increased towards its maximum value according to an exponential function (Model IB) improved the fit considerably (RMSe ≈ 0.013). However, the activation time constant (τ = 30 ms) derived in the fit was too high to reflect Ca2+ binding to troponin. In a further developed model (model II) both Ca2+-binding to troponin and cross-bridge attachment were assumed to be required for full activation. This more complex model gave a good fit to the experimental data (RMSe ≈ 0.013) with a realistic time constant for Ca2+ binding to troponin (9 ms). In both model IB and model II the best fit was obtained with F + ∼ 40 s−1 . An extended version of model IB, with distributed cross-bridge attachment and a series elastic element, gave a fit of similar quality (RMSe ≈ 0.009) as obtained with model IB and model II and with a similar value of F+. The results support the view that weakly bound cross-bridges (state A w) may account for the lead of cross-bridge movement over force during tension rise. It is also shown that, if the stiffness of the myofilaments is non-linear (stiffness increasing with tension) the experimentally observed lead of S(t) over T(t) may, to a significant degree, be attributed to cross-bridges in the state A w.
Similar content being viewed by others
References
Bagni MA, Cecchi G, Cecchini E, Colombini B and Colomo F (1998) Force responses to fast ramp stretches in stimulated frog skeletal muscle fibres. J Muscle Res Cell Mot 19: 33–42.
Bagni MA, Cecchi G, Colomo F and Tesi C (1988a) The mechanical characteristics of the contractile machinery at different levels of activation in intact single muscle fibres of the frog. In: Sugi H and Pollack G (eds.) Molecular Mechanism of Muscle Contraction. (pp. 473–488) Plenum Publishing Corp., New York.
Bagni MA, Cecchi G and Schoenberg M (1988b) A model of force production that explains the lag between crossbridge attachment and force after electrical stimulation of striated muscle fibers. Biophys J 54: 1105–1114.
Baylor SM and Oetliker H (1977) The optical properties of birefrin gence signals from single muscle fibres. J PhysioI 264: 163–198.
Baylor SM, Chandler WK and Marshall MW (1984) Calcium release and sarcoplasmic reticulum membrane potential in frog skeletal muscle fibres. J Physiol 348: 209–238.
Brenner B (1988) Effect of Ca2+on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: Implications for regulation of muscle contraction. Proc Natl Acad Sci USA 85: 3265–3269.
Brenner B (1990) Muscle mechanics and biochemical kinetics. In: Squire JM (ed.) Molecular Mechanisms in Muscular Contraction. (pp. 77–149) Macmillan Press Ltd., New York.
Brenner B, Schoenberg M, Chalovich JM, Greene LE and Eisenberg E (1982) Evidence for cross-bridge attachment in relaxed muscle at low ionic strength. Proc Natl Acad Sci USA 79: 7288–7291.
Bressler BH and Clinch NF (1974) The compliance of contracting skeletal muscle. J Physiol (Lond) 237: 477–493.
Campbell K (1997) Rate constant of muscle force redevelopment reflects cooperative activation as well as cross-bridge kinetics. Biophys J 72: 254–262.
Caputo C, Edman KAP, Lou F and Sun Y-B (1994) Variation in myoplasmic Ca2+ concentration during contraction and relaxation studied by the indicator fluo-3 in frog muscle fibres. J Physiol (Lond) 478: 137–148.
Comincioli V, Torelli A, Poggesi C and Reggiani C (1984) A four-state cross bridge model for muscle contraction. Mathematical study and validation. J Math Biology 20: 277–304.
Cooke R (1997) Actomyosin interaction in striated muscle. Physiol Rev 77: 671–697.
Cecchi G, Colomo F, Lombardi V and Piazzesi G (1987) Stiffness of frog muscle fibres during rise of tension and relaxation in fixed-end or length-clamped tetani. Pflügers Arch 409: 39–46.
Cecchi G, Griffiths PJ, Bagni MA, Ashley CC and Maeda Y (1991) Time-resolved changes in equatorial X-ray diffraction and stiffness during rise of tetanic tension in intact length-clamped single muscle fibers. Biophys J 59: 1273–1283.
Cecchi G, Griffiths PJ and Taylor S (1982) Muscular contraction: kinetics of crossbridge attachment studied by high-frequency stiffness measurements. Science 21: 70–72.
Claflin DR, Morgan DL and Julian FJ (1990) Earliest evidence of cross-bridge activity after the stimulation of single skeletal muscle fibers. Biophys J 57: 425–432.
Curtin NA, Gilbert C, Kretzschmar KM and Wilkie DR (1974) The effect of the performance of work on total energy output and metabolism during muscular contraction. J Physiol (Lond) 238: 455–472.
Diaz Banos FG, Bordas J, Lowy J and Svensson A (1996) Small segmental rearrangements in the myosin head can explain force generation in muscle. Biophys J 71: 576–589.
Edman KAP (1980) Depression of mechanical performance by active shortening during twitch and tetanus of vertebrate muscle fibres. Acta Physiol Scand 109: 15–26.
Edman KAP and Flitney FW (1982) Laser diffraction studies of sarcomere dynamics during ‘isometric’ relaxation in isolated muscle fibres of the frog. J Physiol (Lond) 329: 1–20.
Edman KAP, Mansson A and Caputo C (1997) The biphasic force velocity relationship in frog muscle fibres and its evaluation in terms of cross-bridge function. J Physiol (Lond) 503: 141–156.
Edman KAP and Reggiani C (1984) Redistribution of sarcomere length during isometric contraction of frog muscle fibres and its relation to tension creep. J Physiol (Lond) 351: 169–198.
Eisenberg E, Hill TL and Chen Yi-der (1980) Cross-bridge model of muscle contraction. Quantitative analysis. Biophys J 29: 195–227.
Ford LE, Huxley AF and Simmons RM (1981) The relation between stiffness and filament overlap in stimulated frog muscle fibres. J Physiol (Lond) 311: 219–249.
Ford LE, Huxley AF and Simmons RM (1985) Tension transients during steady shortening of frog muscle fibres. J Physiol (Lond) 361: 131–150.
Ford LE, Huxley AF and Simmons RM (1986) Tension transients during the rise of tetanic tension in frog muscle fibres. J Physiol (Lond) 372: 595–609.
Geeves MA and Halsall DJ (1987) Two-step ligand binding and cooperativity. A model to describe the cooperative binding of myosin subfragment I to regulated actin. Biophys J 52: 215–220.
Geeves MA and Lehrer SS (1994) Dynamics of the muscle thin filament regulatory switch: the size of the cooperative unit. Biophys J 67: 273–282.
Goldman YE and Huxley AF (1994) Actin compliance: Are you pulling my chain? Biophys J 67: 2131–2136.
Griffiths PJ, Ashley CC, Bagni MA, Maéda Y and Cecchi G (1993) Cross-bridge attachment and stiffness during isotonic shortening of intact single muscle fibers. Biophys J 64: 1150–1160.
Head JG, Ritchie MD and Geeves MA (1995) Characterization of the equilibrium between blocked and closed states of muscle thin filaments. Eur J Biochem 227: 694–699.
Higuchi H, Yanagida T and Goldman YE (1995) Compliance of thin filaments in skinned fibers of rabbit skeletal muscle. Biophys J 69: 1000–1010.
Hill TL (1974) Theoretical formalism for the sliding filament model of contraction of striated muscle. Part I. Prog Biophys Mol Biol 28: 267–340.
Holmes KC (1995) The actomyosin interaction and its control by tropomyosin of thin filaments in skinned fibers of rabbit skeletal muscle. Biophys J 68: 2s-7s.
Homsher E (1987) Muscle enthalpy production and its relationship to actomyosin ATPase. Ann Rev Physiol 49: 673–690.
Huxley AF (1957) Muscle structure and theories of contraction. Prog Biophys Biophys Chem 7: 255–318.
Huxley AF and Tideswell S (1996) Filament compliance and tension transients in muscle. J Muscle Res Cell Motil 17: 507–511.
Huxley AF and Simmons RM (1971) Proposed mechanism of force generation in striated muscle. Nature 233: 533–538.
Huxley HE (1975) The structural basis of contraction and regulation in skeletal muscle. Acta Anat Nippon 50: 310–325.
Huxley HE, Faruqi AR, Kress M, Bordas J and Koch MHJ (1982) Time-resolved X-ray diffraction studies of the myosin layer-line reflections during muscle contraction. J Mol Biol 158: 637–684.
Huxley HE, Stewart A, Sosa H and Irving T (1994) X-ray diffraction measurements of the extensibility actin and myosin filaments in contracting muscle. Biophys J 67: 2411–2421.
Irving M (1993) Birefringence changes associated with isometric contraction and rapid shortening steps in frog skeletal muscle fibres. J Physiol (Lond) 472: 127–156.
Irving M, Månsson A, Simmons RM, Piazzesi G, Lombardi V, Ferenczi MA and Harries J (1991) Equatorial X-ray diffraction, stiffness and force time courses during segment length-clamp of tetanized intact fibres isolated from Rana Temporaria muscle. J Physiol (Lond) 438: 147p.
Julian FJ (1969) Activation in a skeletal muscle contraction model with a modification for insect fibrillar muscle. Biophys J 9: 547–570.
Jung DWG, Blangé T, de Graaf H and Treijtel BW (1989) Weakly attached cross-bridges in relaxed frog muscle fibers. Biophys J 55: 605–619.
Jung DWG, Blangé T, de Graaf H and Treijtel BW (1992) Cross bridge stiffness in Ca2+-activated skinned muscle fibres. Pflügers Arch 420: 434–445.
Kaplan W (1981) Advanced Mathematics for Engineers. Addison Wesley Publishing Co., Reading, Massachusetts.
Kodama T (1985) Thermodynamic analysis of muscle ATPase mechanisms. Physiol Rev 65: 467–551.
Kojima H, Ishijima A and Yanagida T (1994) Direct measurement of stiffness of single actin filaments with and without tropomyosin by in vitro nanomanipulation. Proc Natl Acad Sci USA 91: 12962–12966.
Kress M, Huxley HE, Faruqi AR and Hendrix J (1986) Structural changes during activation of frog muscle studied by time-resolved X-ray diffraction. J Mol Biol 188: 325–342.
Lehrer SS (1994) The regulatory switch of the muscle thin filament: Ca2+ or myosin heads? J Muscle Res Cell Motil 15: 232–236.
Lehrer SS and Geeves MA (1998) The muscle thin filament as a classical cooperative/allosteric regulatory system. J Mol Biol 277: 1081–1089.
Lenart TD, Murray JM, Franzini-Armstrong C and Goldman YE (1996) Structure and periodicities of cross-bridges in relaxation, in rigor, and during contractions initiated by photolysis of caged Ca2+. Biophys J 71: 2289–2306.
Malinchik S, Xu S and Yu LC (1997) Temperature-induced structural changes in the myosin thick filament of skinned rabbit psoas muscle. Biophys J 73: 2304–2312.
Malinchik S and Yu LC (1995) Analysis of equatorial X-ray diffraction patterns from muscle fibers: factors that affect the intensities. Biophys J 68: 2023–2031.
Matsubara I and Yagi N (1978) A time-resolved X-ray diffraction study of muscle during twitch. J Physiol (Lond) 278: 297–307.
Maytum R, Lehrer SS and Geeves MA (1999) Cooperativity and switching within the three-state model of muscle regulation. Biochemistry 38: 102–1110.
Mckillop DFA and Geeves MA (1993) Regulation of the interaction between actin and myosin subfragment I: evidence for three states of the thin filament. Biophys J 65: 693–701.
Mijailovich SM, Fredberg JJ and Butler JP (1996) On the theory of muscle contraction: filament extensibility and the development of isometric force and stiffness. Biophys J 71: 1475–1484.
Millar NC and Homsher E (1990) The effect of phosphate and calcium on force generation in glycerinated rabbit skeletal muscle fibers. A steady-state and transient kinetic study. J Bioi Chem 265: 20234–20240.
Månsson A and Edman KAP (1985) Effects of amrinone on the contractile behaviour of frog striated muscle fibres. Acta Physiol Scand 125: 481–493.
Månsson A (1991) The relation between force and stiffness during the rising phase of a tetanus in single muscle fibres of the frog. Abstract of the London Muscle Conference.
Månsson A (1999) Cross-bridge attachment and stiffness during rise of isometric tension in skeletal muscle. J Muscle Res Cell Motil 20: 330.
Peckham M and Irving M (1989) Myosin crossbridge orientation in demembranated muscle fibres studied by birefringence and X-ray diffraction measurements. J Mol Biol 210: 113–126.
Robertson SP, Johnson JD and Potter JD (1981) The time-course of Ca2+ exchange with calmodulin, troponin, parvalbumin, and myosin in response to transient increases in Ca2+. Biophys J 34: 554–569.
Schoenberg M, Brenner B, Chalovich JM, Greene LE and Eisenberg E (1984) Cross-bridge attachment in relaxed muscle. In: Sugi H and Pollack GH (eds.) Contractile Mechanisms in Muscle. pp. (269–284) Plenum Publishing Corp., New York.
Tawada K and Kimura M (1984) Stiffness of glycerinated rabbit psoas fibers in the rigor state: filament-overlap relation. Biophys J 45: 593–602.
Tawada K and Kimura M (1986) Stiffness of carbodiimide-crosslinked glycerinated muscle fibres in rigor and relaxing solutions at high salt concentrations. J Muscle Res Cell Motil 7: 339–350.
Wahr PA and Metzger, JM (1999) Role of Ca2+ and cross-bridges in skeletal muscle thin filament activation probed with Ca2+ sensitizers. Biophys J 76: 2166–2176.
Wakabayashi K, Sugimoto Y, Tanaka H, Deno Y, Takezawa Y and Amemiya Y (1994) X-ray diffraction evidence for the extensibility of actin and myosin filaments during muscle contraction. Biophys J 67: 2422–2435.
Walker JW, Lu Z and Moss RL (1992) Effects of Ca2+ on the kinetics of phosphate release in skeletal muscle. J Bioi Chern 267: 2459–2466.
Xu S, Malinchik S, Gilroy D, Kraft Th, Brenner B and Yu LC (1997) X-ray diffraction studies of cross-bridges weakly bound to actin in relaxed skinned fibers of rabbit psoas muscle. Biophys J 73: 2292–2303.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Månsson, A. Cross-bridge movement and stiffness during the rise of tension in skeletal muscle – a theoretical analysis. J Muscle Res Cell Motil 21, 383–403 (2000). https://doi.org/10.1023/A:1005682712789
Issue Date:
DOI: https://doi.org/10.1023/A:1005682712789