Abstract
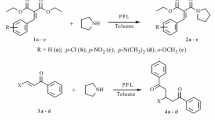
During the enzyme-catalyzed deacylation of O-acylated mandelates with an alcohol, Chromobacterium viscosum lipase was found to catalyze the transesterifications not only at the hydroxy site but also at the carboxyl site of mandelic acids, while such a side-reaction at the carboxyl site was negligible with the other lipases examined.
Similar content being viewed by others
References
Akita H, Matsukura H, Oishi T (1986) Lipase catalyzed enantioselective hydrolysis of 2-methyl 3-acetoxy esters. Tetrahedron Lett. 27: 5241–5244.
Chen C-S, Fujimoto Y, Girdaukas G, Sih CJ (1982) Quantitative analyses of biochemical kinetic resolutions of enantiomers. J. Am. Chem. Soc. 104: 7294–7299.
Chênevert R, Létourneau M (1990) Enantioselectivity of carbonic anhydrase catalyzed hydrolysis of mandelic methyl esters. Can. J. Chem. 68: 314–316.
Coppola GM, Schuster HF (1997) α-Hydroxy Acids in Enantioselective Syntheses, Weinheim: VCH, pp. 137–165.
Feichter C, Faber K, Griengl H (1989) Biocatalytic resolution of long-chain 3-hydroxyalkanoic esters. Tetrahedron Lett. 30: 551–552.
Miyazawa T, Kurita S, Ueji S, Yamada T (1999a) Resolution of mandelic acids by lipase-catalyzed transesterifications in organic media. Biocatal. Biotrans. 16: in press.
Miyazawa T, Shimaoka M, Yamada T (1999b) Resolution of 2-cyano-2-methylalkanoic acids via porcine pancreatic lipase-catalyzed enantioselective ester hydrolysis: effect of the alcohol moiety of the substrate ester on enantioselectivity. Biotechnol. Lett. 21: 309–312.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Miyazawa, T., Kurita, S., Ueji, S. et al. Chromobacterium viscosum lipase catalyzes the transesterifications at the carboxyl site as well as the hydroxy site during the enzymatic enantioselective alcoholysis of O-acylated mandelates. Biotechnology Letters 21, 1023–1027 (1999). https://doi.org/10.1023/A:1005650600997
Issue Date:
DOI: https://doi.org/10.1023/A:1005650600997