Abstract
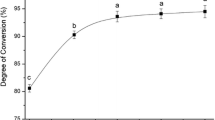
The activity of immobilised soybean lipoxygenase-1 (LOX-1) was studied in aqueous and supercritical carbon dioxide (SCCO2) media for the production of 13S-hydroperoxyoctadecadenoic acid (13S-HPODE). In SCCO2, it was optimal at 33 °C and 25 MPa. A higher space-time yield of 5.7×10−3 Ms−1 mg−1 LOX-1 for 13S-HPODE was obtained in SCCO2 compared to only 5×10−5 Ms−1 mg−1 LOX-1 in aqueous medium. The stability of immobilised LOX-1 was only significantly affected by the pressurisation and depressurisation steps during reactions in SCCO2.
Similar content being viewed by others
References
Aage F, Sather GA (1970) Gas-liquid equilibrium of the oxygencarbon dioxide system. J. Chem. Eng. Data 15: 17–22.
Affleck R, Xu ZF, Susawa V, Focht K, Clark DS, Dordick JS (1992) Enzymatic catalysis and dynamics in low-water environments. Proc. Natl. Acad. Sci. USA 89: 1100–1104.
Berry H, Debat H, Larreta-Garde V (1997) Excess substrate inhibition of soybean lipoxygenase-1 is mainly oxygen dependent. FEBS Lett. 408: 324–326.
Burke PA, Griffin RG Klibanov AM (1992) Solid-state NMR assessment of enzyme active centre structure under non-aqueous conditions. J. Biol. Chem. 267: 20057–20064.
Calvo AM, Hinze LL, Gardner HW, Keller NP (1999) Sporogenic effect of polyunsaturated fatty acids on development of Aspergillus sp. Appl. Environ. Microbiol. 65: 3668–3673.
Christopher JP, Pistorius EK, Regnier FE, Axelrod B (1972) Factors influencing the positional specificity of soybean lipoxygenase. Biochim. Biophys. Acta 289: 82–87.
Darrow AR, Organisciak DT (1994) An improved spectrophotometric tri-iodide assay for lipid hydroperoxides. Lipids 29: 591–594.
Drazen JM, Israel E, O'Bryrne PM(1995) Treatment of asthma with drugs modifying the leukotriene pathway. NewEngl. J. Med. 340: 197–206.
Drouet JM, Legoy MD (1994) Production of 13(S)-hydroxy-9(Z),11(E)-octadecadenoic acid using soybean LOX-1 in a biphasic octane-water system. Tetrahedron Lett. 35: 3923–3926.
Dumont T, Barth D (1992) Enzymatic reaction kinetic: comparison in an organic solvent and in supercritical carbon dioxide. Biotechnol. Bioeng. 39: 329–333.
El-Saadani M, Esterbauer H, El-Sayeed M, Goher M, Nassar AY, Jürgens G (1989) A spectrophotometric assay for lipid peroxides in serum lipoproteins using a commercially available reagent. J. Lipid Res. 30: 627–631.
Feussner I, Porzel A, Wqasternack C (1997) Quantitative Analyse von Lipoxygenase-Metaboliten in Lipiden durch NMRMikroskopie. Biospektrum 3: 54–58.
Galliard T, Chan HWS (1987) Lipoxygenases. In: Stumpf P, Conn EE, eds. The Biochemistry of Plants, Vol. 4. London: Academic Press Inc., pp. 132–157.
Galliard T, Matthew JA (1976) The enzymic formation of long chain aldehydes and alcohols by alpha-oxidation of fatty acids in extracts of cucumber fruit (Cucumis sativus). Biochim. Biophys. Acta 424: 26–35.
Galliard T, Phillips DR, Matthew JA (1975) Enzymatic reactions of fatty acid hydroperoxides in extracts of potato tuber. II. Conversion of 9-and 13-hydroperoxy-octadecadienoic acids to monohydroxydienoic acid, epoxyhydroxy-and trihydroxymonoenoic acid derivatives. Biochim. Biophys. Acta 409: 157–171.
Galunsky B, Schlothhauer R, Böckle B, Kasche V (1994) Direct spectrophotometric measurement of enzyme activity in heterogeneous systems with insoluble substrates or immobilised enzyme. Anal. Biochem. 221: 213–214.
Gardner HW (1996) Lipoxygenase as a versatile biocatalyst. J. Am. Oil Chem. Soc. 73: 1347–1357.
Gardner HW (1997) Analysis of Plant Lipoxygenase Metabolites. Dundee Oily Press Ltd., pp. 1–35.
Gardner HW (1999) Recent investigations into the lipoxygenase pathway of plants. Biochim. Biophys. Acta 1084: 221–239.
Gargouri M, Drouet P, Hervagault JF, Legoy MD (1996) Investigating the behaviour of an enzyme in a bi-phasic system: soybean lipoxygenase-1. Biotechnol. Bioeng. 51: 573–580.
Gießauf A, Magor W, Steinberger DJ, Marr R (1999) A study of hydrolases stability in supercritical carbon dioxide (SCCO2). Enzyme Microbiol. Technol. 24: 577–583.
Ikushima Y, Saito N, Yokohama T, Hatakeda K, Ito S, Arai M, Blanch HW (1992) Solvents effects on an enzymatic ester synthesis in supercritical CO2. Chem. Lett.: 109–112.
Israel E, Cohn J, Drazen JM (1996) Effect of treatment with Zileuton, a 5-LOX inhibitor, in patients with asthma (a randomised controlled trial). J. Am. Med. Assoc. 275: 931–936.
Kachalova GS, Morozov VN, Morozova TY, Myachin ET, Vagin AA, Strokopytov BV, Nekrasov YuV (1991) Comparison of structures of dry and wet hen egg-white lysozyme molecule at 1.8 Å resolution. FEBS Lett. 284: 91–94.
Kamat SV, Iwaskekewycz B, Beckman EJ, Russel AJ (1993) Biocatalytic synthesis of acrylates in supercritical fluids: tuning enzyme activity by changing pressure. Proc. Natl. Acad. Sci. USA 90: 2940–2944.
Kasche V, Schlothauer R, Brunner G (1988) Enzyme denaturation in SCCO2: stabilisation effects of S-S bonds during the depressurisation steps. Biotechnol. Lett. 10: 569–574.
Lozano P, Avellaneda A, Pascual R, Iborra J (1999) Stability of immobilised α-chymotrypsin in supercritical carbon dioxide. Biotechnol. Lett. 18: 1345–1350.
Marty A, Chulalaksananukul W, Condoret JS, Willmot RM, Durand G (1990) Comparison of lipase-catalysed esterification in supercritical carbon dioxide and in n-hexane. Biotechnol. Lett. 12: 11–16.
Nakaruma K (1990) Biochemical reactions in supercritical fluids. Trends Biotechnol. 8: 288–292.
Nakumura K, Min Chi Y, Yamada, Y, Yano T (1986) Lipase activity and stability in supercritical carbon dioxide. Chem. Eng. Commun. 45: 207–212.
Overmeyer A, Schrader-Lippelt S, Kasche V, Brunner G (1999) Lipase catalysed kinetic resolution of racemates at temperatures from 40°C to 160°C in supercritical carbon dioxide. Biotechnol. Lett. 21: 65–69.
Russell AJ, Beckman EJ, Chaudhary AK (1994) Studying enzyme activity in supercritical fluids. Chem. Tech.-Leipzig 24: 33–37.
Veldink GA, Vliegenthart JF, Boldingh J (1977) Plant lipoxygenases. Progr. Chem. Fats Lipids 15: 131–166.
Whitehead I, Muller BL, Dean C (1995) Industrial use of soybean lipoxygenase for the production of natural green note flavour compounds. Cereals Food. World 40: 193–197.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chikere, A., Galunsky, B., Overmeyer, A. et al. Activity and stability of immobilised soybean lipoxygenase-1 in aqueous and supercritical carbon dioxide media. Biotechnology Letters 22, 1815–1821 (2000). https://doi.org/10.1023/A:1005626806321
Issue Date:
DOI: https://doi.org/10.1023/A:1005626806321