Abstract
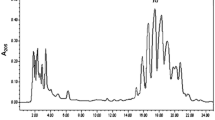
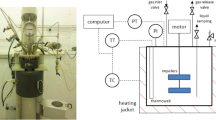
The transesterification of phytosterol and soybean oil was performed using Novozym 435 in supercritical carbon dioxide (SC-CO2). The transesterification reaction was conducted in soybean oil containing 5–25 % phytosterol at 55–95 °C and free-water solvent. The effects of temperature, reaction time, phytosterol concentration, lipase dosage and reaction pressure on the conversion rate of transesterification were investigated. The optimal reaction conditions were the reaction temperature (85 °C), reaction time (1 h), phytosterol concentration (5 %), reaction pressure (8 Mpa) and lipase dosage (1 %). The highest conversion rate of 92 % could be achieved under the optimum conditions. Compared with the method of lipase-catalyzed transesterification of phytosterol and soybean oil at normal pressure, the transesterification in SC-CO2 reduced significantly the reaction temperature and reaction time.





Similar content being viewed by others
Change history
27 April 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00449-021-02575-x
References
Lees AM, Mok HY, Lees RS, McCluskey MA, Grundy SM (1977) Plant sterols as cholesterol-lowering agents: clinical trials in patients with hypercholesterolemia and studies of sterol balance. Atherosclerosis 28:325–338
Wester I (2000) Cholesterol-lowering effect of plant sterols. Eur J Lipid Sci Technol 102:37–44
Katan MB, Grundy SM, Jones P, Law M, Miettinen T, Paoletti R (2003) Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. Mayo Clin Proc 78:965–978
Miettinen TA, Gylling H (2004) Plant stanol and sterol esters in prevention of cardiovascular diseases. Ann Med 36:126–134
Jones PJH, MacDougall DE, Ntanios F, Vanstone CA (1997) Dietary phytosterols as cholesterol-lowering agents in humans. Can J Physiol Pharmacol 75:217–227
Law M (2000) Plant sterol and stanol margarines and health. Br Med J 320:861–864
Neil HAS, Meijer GW, Roe LS (2001) Randomised controlled trial of use by hypercholesterolaemic patients of a vegetable oil sterolenriched fat spread. Atherosclerosis 156:329–337
Weststrate JA, Meijer GW (1998) Plant sterol-enriched margarines and reduction of plasma total and LDL-cholesterol concentrations in normocholesterolaemic and mildly hypercholesterolaemic subjects. Eur J Clin Nutr 52:334–343
Ling WH, Jones PJH (1995) Dietary phytosterols: a review of metabolism, benefits and side effects. Life Sci 57:195–206
Lilin L, Wei D, Dehua L, Li W, Zebo L (2006) Lipase-catalyzed transesterification of rapeseed oils for biodiesel production with a novel organic solvent as the reaction medium. J Mol Catal B Enzym 43:58–62
Zeng CX, Qi SJ, Li ZG, Luo RM (2015) Enzymatic synthesis of phytosterol esters catalyzed by Candida rugosa lipase in water-in-[Bmim]PF6 microemulsion. Bioprocess Biosyst Eng 38:939–946
Li Z, Yang DP, Jiang L, Ji JF (2007) Lipase-catalyzed esterification of conjugated linoleic acid with l-carnitine in solvent-free system and acetonitrile. Bioprocess Biosyst Eng 30:331–336
Negishi S, Hidaka I, Takahashi I, Kunita S (2003) Transesterification of phytosterol and edible oil by lipase powder at high temperature. J Am Oil Chem Soc 80:905–907
Villeneuve P, Turon F, Caro Y, Escoffier R, Barea B, Barouh B (2005) Lipase-catalyzed synthesis of canola phytosterols oleate esters as cholesterol lowering agents. Enzyme Microb Technol 37:150–155
Vu PL, Shin JA, Lim CH, Lee KT (2004) Lipase-catalyzed production of phytosteryl esters and their crystallization behavior in corn oil. Food Res Int 37:175–180
Weber N, Weitkamp P, Mukherjee KD (2002) Cholesterollowering food additives: lipase-catalysed preparation of phytosterol and phytostanol esters. Food Res Int 235:177–181
Jackson MA, King JW, List GR, Neff WE (1997) Lipase-catalyzed randomization of fats and oils in flowing supercritical carbon dioxide. J Am Oil Chem Soc 74:635–639
Gunnlaugsdottir H, Järemo M, Sivik B (1998) Process parameters influencing ethanolysis of cod liver oil in supercritical carbon dioxide. J Supercrit Fluids 12:85–93
King JW (2000) Advances in critical fluid technology for food processing. Food Sci Technol Today 14(4):186–191
Weber N, Weitkamp P, Mukherjee KD (2002) Cholesterol-lowering food additives: lipase-catalysed preparation of phytosterol and phytostanol esters. Food Res Int 35(2–3):177–181
Weber N, Weitkamp P, Mukherjee KD (2001) Steryl and stanyl esters of fatty acids by solvent-free esterification and transesterification in vacuo using lipases from Rhizomucor miehei, Candida antarctica, and Carica papaya. J Agric Food Chem 49:5210–5216
Venskutonis PR, Daukšas E, Sivik B (2008) Use of immobilised lipase from Candida antarctica in supercritical fluid extraction of borage seed Oil. Food Technol Biotechnol 46(2):157–163
Acknowledgments
This work was supported by a grant from the Heilongjiang Provincial Basic Scientific Research Business Foundation: Synthesis of phytosteryl esters by esterification and transesterification in vacuo and without solvent using lipases (No: DADOU2014-2). This work was supported by a grant from the National Natural Science Foundation of China (NSFC): Study on the method of controlling TFAs in oil by orientated hydrogenation and mechanism of molecular reaction under CO2 supercritical state (No: 31271886). This work was also supported by a grant from the Key Laboratory of Soybean Biology in Chinese Ministry of Education Northeast Agricultural University, Harbin, China, 150030. The authors are also grateful to the anonymous referees and the editor for helpful comments on an earlier draft.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hu, L., Llibin, S., Li, J. et al. Lipase-catalyzed transesterification of soybean oil and phytosterol in supercritical CO2 . Bioprocess Biosyst Eng 38, 2343–2347 (2015). https://doi.org/10.1007/s00449-015-1469-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-015-1469-5