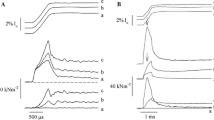
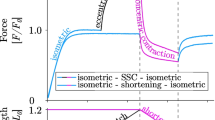
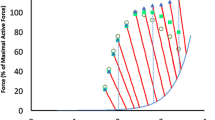
Abstract
The tension rise during stretch of passive skeletal muscle is biphasic, with an initial steep rise, followed by a subsequent more gradual change. The initial rise has been interpreted as being due to the presence of numbers of long-term, stable cross-bridges in resting muscle fibres. A point of weakness with the cross-bridge interpretation is that the initial stiffness reaches its peak value at muscle lengths beyond the optimum for myofilament overlap. To explain this result it has been suggested that despite the reduced overlap at longer lengths, the closer interfilament spacing and a higher sensitivity of the myofilaments to Ca2+ allows more stable cross-bridges to form. Recently the stretch responses of passive muscle have been re-examined and it has been suggested that it is not necessary to invoke cross-bridge mechanisms at all. Explanations based on a viscous resistance to interfilament sliding and mechanical properties of the elastic filaments, the gap filaments, were thought to adequately account for the observed tension changes. However, an important property of passive muscle, the dependence of stretch responses on the immediate history of contraction and length changes, thixotropy, cannot be explained simply in terms of viscous and viscoelastic properties. The review discusses the cross-bridge interpretation of muscle thixotropy and the relationship of passive stiffness to filament resting tension and latency relaxation. It is proposed that cross-bridges can exist in three states; one, responsible for the resting stiffness, requires resting levels of calcium. When, during activation, calcium levels rise, cross-bridges enter a low-force, high-stiffness state, signalled by latency relaxation, before they move to the third, force-generating state. It is concluded that, compared with viscoelastic models, a cross-bridge-based explanation of passive muscle properties is better able to accommodate the currently known facts although, as new information becomes available, this view may need to be revised.
Similar content being viewed by others
References
Bagni MA, Cecchi G, Colomo F and Garzella P (1992) Are weakly binding bridges present in resting intact muscle fibres? Biophys J 63: 1412-1415.
Bagni MA, Cecchi G, Colomo F and Garzella P (1995) Absence of mechanical evidence for attached weakly binding cross-bridges in frog relaxed muscle fibres. J Physiol 482: 391-400.
Balnave CD and Allen DG (1996) The effect of muscle length on intracellular calcium and force in single fibres from mouse skeletal muscle. J Physiol 492: 705-713.
Bartoo ML, Linke WA and Pollack GH (1997) Basis of passive tension and stiffness in isolated rabbit myofibrils. Am J Physiol 273: C266-C276.
Bershitsky SY, Tsturyan AK, Bershitskaya ON, Mashanov GI, Brown P, Burns R and Ferenczi MA (1997) Muscle force is generated by myosin heads stereospecifically attached to actin. Nature 388: 186-190.
Brenner B, Schoenberg M, Chalovich JM, Greene LE (1982) Muscle mechanism and biochemical kinetics. In: Squire JM (ed). Molecular Mechanism in Muscular Contraction (pp. 77-149). Macmillan, Southampton.
Campbell KS and Lakie M (1998) A cross-bridge mechanism can explain the thixotropic short-range elastic component of relaxed frog skeletal muscle. J Physiol 510: 941-962.
Chalovich JM, Chock PB and Eisenberg E (1981) Mechanism of action of troponin-tropomyosin inhibition of actomyosin ATPase activity without inhibition of myosin binding to actin. J Biol Chem 256: 575-578.
Claflin DR, Morgan DL and Julian FJ (1990) Earliest mechanical evidence of cross-bridge activity after stimulation of single skeletal muscle fibres. Biophys J 57: 425-432.
Claflin DR, Morgan DL, Stephenson DG and Julian FJ (1994) The intracellular Ca2+ transient and tension in frog skeletal muscle fibres measured with high temporal resolution. J Physiol 475: 319-325.
Close RI (1981) Activation delays in frog twitch muscle fibres. J Physiol 313: 81-100.
Close RI and Lännergren JI (1984) Arsenazo III calcium transients and latency relaxation in frog skeletal muscle fibres at different sarcomere lengths. J Physiol 355: 323-344.
Denny-Brown D (1929) On the nature of postural reflexes. Proc R Soc Lond Ser B 104: 252-301.
Endo M (1973) Length dependence of activation of skinned muscle fibres by calcium. Cold Spring Harbor Symp Quant Biol 37: 505-510.
Ford LE, Huxley AF and Simmons RM (1977) Tension response to sudden length change in stimulated frog muscle fibres near slack length. J Physiol 269: 441-515.
Ford LE, Huxley AF and Simmons RM (1981) The relation between stiffness and filament overlap in stimulated frog muscle fibres. J Physiol 311: 219-249.
Goldspink G (1985) Malleability of the motor system: a comparative approach. J Exp Biol 115: 375-391.
Goldspink G (1999) Changes in muscle mass and phenotype and the expression of autocrine and systemic growth factors by muscle in response to stretch and overload. J Anat 194: 1-12.
Gonzalez-Serratos H (1971) Inward spread of activation in vetebrate muscle fibres. J Physiol 212: 777-799.
Haugen P (1982) The dependence of the latency relaxation on temperature in single muscle fibres of the frog. Acta Physiol Scand 114: 179-186.
Haugen P and Sten-Knudsen O (1976) Sarcomere lengthening and tension drop in the latent period of isolated frog skeletal muscle fibres J Gen Physiol 68: 247-265.
Haugen P and Sten-Knudsen O (1981) The dependence of the short-range elasticity on sarcomere length in resting isolated frog muscle fibres. Acta Physiol Scand 112: 113-120.
Hill DK (1968) Tension due to interaction between the sliding filaments in resting striated muscle. The effect of stimulation. J Physiol 199: 637-684.
Huxley AF (1957) Muscle structure and theories of contraction. Prog Biophys Biophys Chem 7: 255-318.
Jolesz F and Sreter FA (1981) Development, innervation, and activity-pattern induced changes in skeletal muscle. Ann Rev Physiol 43: 531-552.
Jones C, Allen T, Talbot J, Morgan DL and Proske U (1997) Changes in the mechanical properties of human and amphibian muscle after eccentric exercise. Eur J Appl Physiol 76: 21-31.
Kellermayer MSZ, Smith SB, Granzier HL and Bustamante C (1997) Folding-unfolding transitions in single titin molecules characterized with laser tweezers. Science 276: 1112-1116.
Lakie M, Walsh EG and Wright GW (1984) Resonance at the wrist demonstrated by the use of a torque motor: an instrumental analysis of muscle tone in man. J Physiol 353: 265-285.
Lännergren J (1971) The effect of low-level activation on the mechanical properties of isolated frog muscle fibers. J Gen Physiol 58: 145-162.
Liddell EGT and Sherrington CG (1925) Further observations on myotatic reflexes. Proc R Soc Lond Ser B 97: 267-283.
Macefield VG, Gandevia SC, Bigland-Ritchie B, Gorman RB and Burke D (1993) The firing rates of human motoneurones voluntarily activated in the absence of muscle afferent feedback. J Physiol 471: 429-443.
Macpherson PCD, Dennis RG and Faulkner JA (1997) Sarcomere dynamics and contraction-induced injury to maximally activated single muscle fibres from soleus muscles of rats. J Physiol 500: 523-533.
Morgan DL (1990) New insights into the behaviour of muscle during active lengthening. Biophys J 57: 209-221.
Morgan DL, Claflin DR and Julian FJ (1990) Tension in frog single muscle fibres while shortening actively and passively at velocites near Vu. Biophys J 57: 1001-1007.
Morgan DL, Prochazka A and Proske U (1984) The after-effects of stretch and fusimotor stimulation on the responses of primary endings of cat muscle spindles. J Physiol 356: 465-477.
Moss RL, Sollins MR and Julian FJ (1976) Calcium activation produces a characteristic response to stretch in both skeletal and cardiac muscle. Nature 260: 619-621.
Mutungi G and Ranatunga KW (1996a) The visco-elasticity of resting intact mammalian (rat) fast muscle fibres. J Muscle Res Cell Motility 17: 357-364.
Mutungi G and Ranatunga KW (1996b) The viscous, viscoelastic and elastic characteristics of resting fast and slow mammalian (rat) muscle fibres. J Physiol 496: 827-836.
Mutungi G and Ranatunga KW (1998) Temperature-dependent changes in the viscoelasticity of intact resting mammalian (rat) fast-and slow-twitch muscle fibres. J Physiol 508: 253-265.
Proske U and Morgan DL (1987) Tendon stiffness: methods of measurement and significance for the control of movement. A review. J Biomechanics 20: 75-82.
Proske U, Morgan DL and Gregory JE (1993) Thixotropy in skeletal muscle and in muscle spindles: A review. Prog Neurobiol 41: 705-721.
Ribot-Ciscar E and Roll J-P (1998) Ago-antagonist muscle spindle inputs contribute together to joint movement coding in man. Brain Res 791: 167-176.
Sandow A (1970) Skeletal muscle. Annu Rev Physiol 32: 87-138.
Stephenson DG and Wendt IR (1984) Length dependence of changes in sarcoplasmic calcium concentration and myofibrillar calcium sensitivity in striated muscle fibres. J Musc Res Cell Motility 5: 243-272.
Tskhovrebova L, Trinick J, Sleep JA and Simmons RM (1997) Elasticity and unfolding of single molecules of the giant muscle protein titin. Nature 387: 308-312.
Walmsley B, Hodgson JA and Burke RE (1978) Forces produced by medial gastrocnemius and soleus muscles during locomotion in freely moving cats. J Neurophysiol 41: 1203-1216.
Walmsley B and Proske U (1981) Comparison of stiffness of soleus and medial gastrocnemius muscles in cats. J Neurophysiol 46: 250-259.
Wang K, Mccarter R, Wright J, Beverly J and Ramirez-Mitchell R (1991) Regulation of skeletal muscle stiffness and elasticity by titin isoforms: a test of the segmental extension model of resting tension. Proc Natl Acad Sci USA 88: 7101-7105.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Proske, U., Morgan, D.L. Do cross-bridges contribute to the tension during stretch of passive muscle?. J Muscle Res Cell Motil 20, 433–442 (1999). https://doi.org/10.1023/A:1005573625675
Issue Date:
DOI: https://doi.org/10.1023/A:1005573625675