Abstract
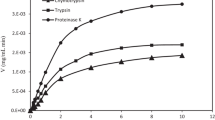
We have previously reported (Kim et al., J. Dairy Res., 1999, in press) three novel angiotensin-I-converting enzyme (ACE) inhibitory peptides, Tyr-Pro-Glu-Arg, Tyr-Tyr-Pro-Gln-Ile-Met-Gln-Tyr and Asn-Asn-Val-Met-Leu-Gln-Trp, isolated from the tryptic digest of recombinant human αs1-casein. A series of C-terminal di- and tripeptides from these three peptides have now been synthesized and their ACE inhibitory activities have been measured. Among the peptides tested, the tripeptide Leu-Gln-Trp had the highest ACE inhibitory activity (IC50=3.8 μmol l−1).
Similar content being viewed by others
References
Adibi SA (1971) Intestinal transport of dipeptides in man: relative importance of hydrolysis and intact absorption. J. Clin. Invest. 50: 2266–2275.
Cavaletto M, Cantisani A, Gluffrida G, Napolitano I, Caen J (1994) Human α s1-casein like protein: purification and N-terminal sequence determination. Biol. Chem. Hoppe-Seyler 375: 149–151.
Cheung HS, Wang FL, Ondetti MA, Sabo EF, Cushman DW (1980) Binding of peptide substrates and inhibitors of angiotensin-converting enzyme. Importance of the COOH-terminal dipeptide sequence. J. Biol. Chem. 255: 401–407.
Fiat AM, Migliore-Samour D, Jolles P, Drouet L, Sollier CB, Caen J (1993) Biologically active peptides from milk proteins with emphasis on two examples concerning antithrombotic and immunomodulating activities. J. Dairy Sci. 76: 301–310.
Hara H, Funabiki R, Iwata M, Yamazaki K (1984) Portal absorption of small peptides in rats under unrestrained conditions. J. Nutr. 114: 1122–1129.
Johnsen LB, Rasmussen LK, Petersen TE, Berglund L (1995) Characterization of three types of human α s1-casein mRNA transcripts. Biochem. J. 209: 237–242.
Kim YK, Chung BH, Yoon S, Lee KK, Lönnerdal B, Yu DY (1997) High-level expression of human α s1-casein in Escherichia coli. Biotechnol. Techn. 11: 675–678.
Kim YK, Yoon S, Yu DY, Lönnerdal B, Chung BH (1999) Novel angiotensin-I-converting enzyme inhibitory peptides derived from recombinant human α s1-casein expressed in Escherichia coli. J. Dairy Res. (in press).
Laragh JH, Bear L, Brunner HR, Buhler FG, Sealey JE, Vaughan ED (1972) Rennin angiotensin and aldosterone system in pathogenesis and management of hypertensive vascular disease. Am. J. Med. 52: 633–652.
Rasmussen LK, Due HA, Petersen TE (1995) Human α s1-casein: purification and characterization. Comput. Biochem. Physiol. 111B: 75–81.
Schlimme E, Meisel H (1995) Bioactive peptides derived from milk proteins: structural, physiological and analytical aspects. Die Nahrung 1: 1–20.
Skeggs LT, Kahn JE, Shumway, NP (1956) The preparation and function of the angiotensin-converting enzyme. J. Exp. Med. 103: 295–299.
Wyvratt MJ, Patchett AA (1985) Recent developments in the design of angiotensin-converting enzyme inhibitors. Med. Res. Rev. 5: 485–531.
Yamamoto N (1997) Antihypertensive peptides derived from food proteins. Biopolymers 43: 129–134.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kim, Y.K., Chung, B.H. A novel angiotensin-I-converting enzyme inhibitory peptide from human αs1-casein. Biotechnology Letters 21, 575–578 (1999). https://doi.org/10.1023/A:1005572504196
Issue Date:
DOI: https://doi.org/10.1023/A:1005572504196