Abstract
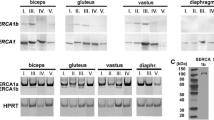
Members of the calpain proteinase family are present in all mammalian cells, although a novel calpain 94kDa isoform is found almost exclusively in skeletal muscle. p94 is difficult to purify from muscle and recombinant p94 autolyses rapidly when expressed in COS cells. However, in vivo the enzyme may be stabilised by interaction with titin, which has two well-characterised binding sites for p94 at the N2- and M-lines. Both these titin subdomains are subject to muscle-specific alternative splicing, which could be related to p94 expression level or stability in muscles of different fibre type. In this study, porcine longissimus dorsi (LD), trapezius (TZ) and adductor longus (AL) were characterised as fast, intermediate and slow using commercially available specific anti-human fast- and slow-myosin heavy chain mAbs and also by conventional histochemistry. p94 was quantified both in whole muscle preparations and single fibres by western blotting using an anti-p94 antiserum generated by expressing a recombinant p94 sequence as a GST fusion protein antigen.
SDS PAGE and immunoblotting revealed a single band of approximately 94kDa with identical mobility in all muscle and fibre preparations. The intensity of the 94 kDa band was greater in LD (22 ± 1.7 densitometric units mean ± SEM, n = 3) than TZ and AL (10 ± 2.3 and 6 ± 0.9 units, respectively). Expressed as a ratio relative to actin immunoreactivity, p94 is present in all types of single fibres isolated from TZ, but at a significantly lower level (P < 0.01) in slow type I (0.08 ± 0.01, n = 9), compared to fast IIA/IIB fibres (0.22 ± 0.02, n = 26). No evidence was seen for rapid or variable rate of p94 degradation in either type of fibre. These data suggest a positive correlation between p94 expression level and fast glycolytic characteristics in porcine muscle.
Similar content being viewed by others
References
Barr A and Pette D (1988) Three fast myosin heavy chains in adult rat skeletal muscle. FEBS Lett 235: 153-155.
Biral D, Betto R, Danieli-Betto D and Salviati G (1988) Myosin heavy chain composition of single fibres from normal human muscle. Biochem J 250: 307-308.
Brooke MH and Kaiser KK (1970) Three ``myosin adenosine triphosphatase'' systems: the nature of their pH lability and sulfhydryl dependence. J Histochem Cytochem 18: 670-672.
Cleveland DW, Fischer SG, Kirschner MW and Laemmli UK (1977) Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem 252: 1102-1106.
Fougerousse F, Durand M, Suel L, Pourquie O, Delezoide AL, Romero NB, Albitbol M and Beckmann JS (1998) Expression of genes (CAPN3, SGCA, SGCB, and TTN) involved in progressive muscular dystrophies during early human development. Genomics 48: 145-156.
Fritz JD, Swartz DR and Greaser ML (1989) Factors a€ecting polyacrylamide gel electrophoresis and electroblotting of high molecular weight myofibrillar proteins. Anal Biochem 180: 205-210.
Goll DE, Thompson VF, Taylor RG and Ouali A (1998) The calpain system and skeletal muscle growth. Can J Anim Sci 78: 503-512.
Gorza L (1990) Identification of a novel type 2 fiber population in mammalian skeletal muscle by combined use of histochemical myosin ATPase and anti-myosin monoclonal antibodies. J Histochem Cytochem 38: 257-265.
Huang J and Forsberg NE (1998) Role of calpain in skeletal-muscle protein degradation. Proc Natl Acad Sci USA 95: 12100-12105.
Kinbara K, Sorimachi H, Ishiura S and Suzuki K (1997) Muscle-specific calpain, p94, interacts with the extreme C-terminal region of connectin, a unique region flanked by two immunoglobulin C2 motifs. Arch Biochem Biophys 342: 99-107.
Kinbara K, Ishiura S, Tomioka S, Sorimachi H, Jeong S-Y, Amano S, Kawasaki H, Kolmerer B, Kimura S, Labeit S and Suzuki K (1998) Purification of native p94, a muscle-specific calpain, and characterization of its autolysis. Biochem J 335: 589-596.
Kolmerer B, Olivieri N, Witt C, Herrmann BG and Labeit S (1996) Genomic organization of M line titin and its tissue-specific expression in two distinct isoforms. J Mol Biol 256: 555-563.
Labeit S and Kolmerer B (1995) Titins: giant proteins in charge of muscle ultrastructure and elasticity. Science 270: 293-296.
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 227: 680-685.
Lefaucheur L, Hoffman RK, Gerrard DE, Okamura CS, Rubinstein N and Kelly A (1998) Evidence for three adult fast myosin heavy chain isoforms in type II skeletal muscle fibres in pigs. J Anim Sci 76: 1584-1593.
Parr T, Sensky PL, Scothern GP, Bardsley RG, Buttery PJ, Wood JD and Warkup C (1999) Relationship between skeletal muscle-specific calpain and tenderness of conditioned porcine longissimus muscle. J Anim Sci 77: 661-668.
Perrie WT and Bumford SJ (1986) Electrophoretic separation of myosin isoenzymes: Implications for the histochemical demonstration of fibre types in biopsy specimens of human skeletal muscle. J Neurol Sci 73: 89-96.
Richard I, Broux O, Allamand V, Fougerousse F, Chiannikulchai N, Bourg N, Brenguier L, Devaud C, Pasturaud P, Roudat C, Hillaire D, Passos-Bueno MR, Zatz M, Tischfield JA, Fardeau M, Jackson CE, Cohen D and Beckmann JS (1995) Mutations in the proteolytic enzyme calpain 3 cause limb girdle muscular dystrophy 2A. Cell 81: 27-40.
Saido TC, Sorimachi H and Suzuki K (1994) Calpain: new perspectives in molecular diversity and hysiological-pathological involvement. FASEB J 8: 814-822.
Schantz PG and Dhoot GK (1987) Coexistence of slow and fast isoforms of contractile and regulatory proteins in human skeletal muscle fibres induced by endurance training. Acta Physiol Scand 131: 147-154.
Sorimachi H, Saido TC and Suzuki K (1994) New era of calpain research-discovery of tissue-specific calpains. FEBS Lett 343: 1-5.
Sorimachi H, Kinbara K, Kimura S, Takahashi M, Ishuira S, Sasagawa N, Sorimachi N, Shimada H, Tagawa K, Maruyama K and Suzuki K (1995) Muscle-specific calpain, p94, responsible for limb girdle muscular dystrophy type 2A, associates with connectin through IS2, a p94-specific sequence. J Biol Chem 270: 31158-31162.
Sorimachi H, Forsberg NE, Lee HJ, Joeng SY, Richard I, Beckmann JS, Ishiura S and Suzuki K (1996a) Highly conserved structure in the promoter region of the gene for muscle specific calpain, p94. J Biol Chem 377: 859-864.
Sorimachi H, Kimura S, Kinbara K, Kazama J, Takahashi M, Yajima H, Ishiura S, Sasagawa N, Nonaka I, Sugita H, Muruyama K and Suzuki K (1996b) Structure and physiological functions of ubiquitous and tissue-specific calpain species. Adv Biophys 33: 101-122.
Spencer MJ, Tidball JG, Anderson LVB, Bushby KMD, Harris JB, Passos-Bueno MR, Somer H, Vainzof M and Zatz M (1997) Absence of calpain 3 in a form of limb-girdle muscular dystrophy (LGMD2A). J Neurol Sci 146: 173-178.
Staron RS (1991) Correlation between myofibrillar ATPase activity and myosin heavy chain composition in single human muscle fibers. Histochemistry 96: 21-24.
Staron RS and Hikida RS (1992) Histochemical, biochemical, and ultrastructural analyses of single human muscle fibers with special reference to the C fiber population. J Histochem Cytochem 40: 563-568.
Staron RS and Pette D (1993) The continuum of pure and hybrid myosin heavy chain based fiber types in rat skeletal muscle. J Histochem 100: 149-153.
Termin A, Staron RS and Pette D (1989) Myosin heavy chain isoforms in histochemically defined fibre types of rat muscle. Histochem 92: 453-457.
Towbin H, Staedlin T and Gordon J (1979) Electrophoretic transfer of protein from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76: 4350-4354.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jones, S.W., Parr, T., Sensky, P.L. et al. Fibre type-specific expression of p94, a skeletal muscle-specific calpain. J Muscle Res Cell Motil 20, 417–424 (1999). https://doi.org/10.1023/A:1005572125827
Issue Date:
DOI: https://doi.org/10.1023/A:1005572125827