Abstract
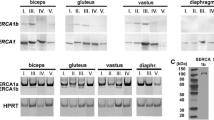
In this study we developed an SDS-PAGE protocol which for the first time separates effectively all myosin heavy chain (MHC) isoforms expected to be expressed in iliofibularis (IF), pyriformis (PYR), cruralis (CRU) and sartorius (SAR) muscles of the toad Bufo marinus on the basis of previously reported fibre type composition. The main feature of the method is the use of alanine instead of glycine both in the separating gel and in the running buffer. The correlation between the MHC isoform composition of IF, SAR and PYR muscles determined in this study and the previously reported fibre type composition of IF and SAR muscles in the toad and of PYR muscle in the frog was used to tentatively identify the MHC isoforms expressed by twitch fibre types 1, 2 and 3 and by tonic fibres. The alanine-SDS electrophoretic method was employed to examine changes in the MHC composition of IF, PYR, CRU and SAR muscles with the ontogenetic growth of the toad from post-natal life (body weight < 1 g) to late adulthood (body weight 200–450 g). The developmental changes in the MHC isoform composition of the toad IF muscle observed in this study are in very good agreement with those in the fibre type composition of the developing IF muscle reported in the literature.
Similar content being viewed by others
References
Bradford M (1976) A rapid and sensitive method for the quantitation of the microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–258.
Chrambach A (1985) The practice of quantitative gel electrophoresis. Weinheim, Deerfield Beach FL.
Freeland WJ and Kerin SH (1991) Ontogenetic alternation of activity and habitat selection by Bufo marinus. Wildl Res 18: 431–443.
Gilly WF (1975) Slow fibres in the frog cruralis muscle. Tissue Cell 7(1): 203–210.
Hämäläinen N and Pette D (1995) Pattern of myosin isoforms in mammalian skeletal muscle fibres. Microsc Res Tech 30: 381–389.
Hochstrasser DF, Patchornik A and Merryl CR (1988) Development of polyacrylamide gels that improve the separation of proteins and their detection by silver staining. Anal Biochem 173: 412–423.
Hoh JFY, Wah MSY, Everett AW and Hughes S (1994) Plasticity of twitch muscle fibres of the toad, Bufo marinus, during adaptations to the seasons and to post metamorphic life. Basic Appl Myol 4: 369–380.
Huxley AF (1974) Muscular contraction. J Physiol 243: 1–43.
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685.
Lännergren J and Hoh JFY (1984) Myosin isoenzymes in single muscle fibres of Xenopus laevis: analysis of five different functional types. Proc R Soc Lond B 222: 401–408.
Lännergren J and Smith RS (1966) Types of muscle fibres in toad skeletal muscle. Acta Physiol Scand 68: 263–274.
Lutz GJ, Cuizon DB, Ryan AF and Lieber RL (1998a) Four novel myosin heavy chain transcripts define a molecular basis for muscle fibre types in Rana pipiens. J Physiol 508: 667–680.
Lutz GJ, Bremmer S, Lajevardi N, Lieber RL and Rome LC (1998b) Quantitative analysis of muscle fibre type and myosin heavy chain distribution in the frog hindlimb: implications for locomotory design. J Muscle Res Cell Motil 19: 717–731.
Martyn DA, Coby R, Huntsman LL and Gordon AM (1993) Force-calcium relations in skinned twitch and slow-tonic frog muscle fibres have similar sacromere length dependencies. J Muscle Res Cell Motil 14: 65–75.
Moss RL, Diffee GM and Greaser ML (1995) Contractile properties of skeletal muscle fibres in relation to myofibrillar protein isoforms. Rev Physiol Biochem Pharmacol 126: 2–63.
Orkand PR, Orkand RK and Cohen MW (1978) Distribution of acetylcholine receptors on Xenopus slow muscle fibres determined by a-bungarotoxin binding. Neuroscience 3: 435–446.
Pette D and Staron RS (1997) Mammalian skeletal muscle type transitions. Int Rev Cytol 170: 143–223.
Reiser PJ and Kline WO (1998) Electrophoretic separation and quantitation of cardiac myosin heavy chain isoforms in eight mammalian species. Am J Physiol 274: H1048–H1053.
Rossini K, Rizzi C, Sandri M, Bruson A and Carraro U (1995) High-resolution sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunochemical identification of the 2X and embryonic myosin heavy chains in complex mixtures of isomyosins. Electrophoresis 16: 101–106.
Rowlerson AM and Spurway NC (1985) How many fibre types in amphibian limb muscle? A comparison of Rana and Xenopus. J Physiol 358: 78P.
Smith RS and Ovalle Jr. WK (1973) Varieties of fat and slow extrafusal muscle fibres in amphibian hind limb muscles. J Anal 116: 1–24.
Sperry DG (1981) Fibre type composition and postmetamorphic growth of anuran hind limb muscles. J Morphol 170: 321–345.
Talmadge RJ and Roy RR (1993) Electrophoretic separation of rat skeletal muscle myosin heavy chain isoforms. J Appl Physiol 75: 2337–2340.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nguyen, L.T., Stephenson, G.M.M. An electrophoretic study of myosin heavy chain expression in skeletal muscles of the toad Bufo marinus. J Muscle Res Cell Motil 20, 687–695 (1999). https://doi.org/10.1023/A:1005560431865
Issue Date:
DOI: https://doi.org/10.1023/A:1005560431865