Abstract
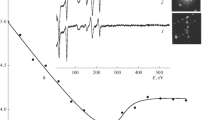
Auger measurements reveal that, under UHV conditions, interfacial sulfurinduces the destabilization of an aluminum oxide overlayer at theFe–Al2O3 interface at temperatures above400 K. One monolayer deposition of Al onto Fe/S results in the insertion ofAl at the Fe–S interface. Exposure of Fe–Al–S to oxygenat 300 K gives rise to the complete oxidation of the aluminum adlayer asevidenced by the disappearance of the Al0 Auger signal and thestoichiometric formation of the aluminum oxide. When the resultingFe–S–Al2O3 is annealed progressively tohigher temperatures between 400 and 900 K, analysis of the Auger spectrashows a dramatic decline in the Al/O Auger intensity ratio. This declineis accompanied by the appearance of a small signal due to Al0,which maintains a constant intensity as the total Al signal (due mainly toAl3+) decreases. The appearance of the Al0 Augersignal accompanied by the attenuation of the Al3+ signalsignifies the chemical conversion of Al3+ into Al0,probably followed by diffusion of Al into the bulk. The possibility ofalumina dewetting and island formation, however, cannot be ruled out onthe basis of the present data. In the absence of interfacial sulfur, the alumina–Fe interface is stable to 900 K.
Similar content being viewed by others
REFERENCES
J. L Smialek, Metall. Trans. 22A, 739 (1991).
J. L. Smialek, D. T. Jane, J. C. Schaeffer, and W. H. Murphy, Thin Solid Films 253, 285 (1994).
J. D. Kiely, T. Yeh, and D. A. Bonnell, Surface Sci. 393, L126 (1997).
P. Y. Hou, Z. Wang, K. Prüssner, K. B. Alexander, and I. G. Brown, Proc. 3rd Intern. Conf. Microsc. Oxid. (Cambridge, U.K., 1996).
P. Y. Hou and J. Stringer, Oxid. Met. 38, 323 (1992).
H. Cabibil and J. A. Kelber, Surface Sci. 329, 101 (1995).
P. Fox, D. G. Lees, and G. W. Lorimer, Oxid. Met. 36, 491 (1991).
J. A. Kelber, S. G. Addepalli, J.-S. Lin, and H. Cabibil, Proc. Electrochem. Soc. Symp. High Temp. Corros. Mater. Chem., San Diego, CA, 3-5 May, 1998, P. Y. Hou, M. J. McNallan, R. Oltra, E. J. Opila, and D. A Shores, eds. (The Electrochemical Society, 1998), pp. 190-197.
J.-S. Lin, B. Ekstrom, S. G. Addepalli, H. Cabibil, and J. A. Kelber, Langmuir 14, 4843 (1998).
S. G. Addepalli, J.-S. Lin, B. Ekstrom, and J. A. Kelber, Oxid. Met. 52, 139 (1999).
C. G. Walker and M. M. El Gomati, Appl. Surface Sci. 35, 164 (1988-89).
H. J. Grabke, D. Wiemer, and H. Viefhaus, Fresenius J. Anal. Chem. 341, 402 (1991).
F. H. Stott, Rep. Prog. Phys. 50, 861 (1987).
A. W. Funkenbusch, J. Smeggil, and N. S. Bornstein, Metall. Trans. 16A, 1164 (1985).
P. Y. Hou and J. Stringer, J. Phys. IV 3, 231 (1993).
S. Y. Hong, A. B. Anderson, and J. L. Smialek, Surface Sci. 230, 175 (1990).
W. Arabczyk, T. Baumann, H.-J. Mussig, and F. Storbeck, Vacuum 41, 79 (1990).
L. E. Davis, N. C. MacDonald, P. W. Palmberg, G. E. Riach, and R. E. Weber, Handbook of Auger Electron Spectroscopy, 2nd ed. (Physical Electronics Industries, Eden Prairie, MN, 1979).
M. P. Seah, in Practical Surface Analysis, 2nd edn., Vol. 1: Auger and X-ray Photoelectron Spectroscopy, D. Briggs and M. P. Seah, eds. (Wiley, New York, 1990), pp. 201-255.
V. S. Smentkowski and J. T. Yates, Jr., Surface Sci. 232, 113 (1990).
G. Ertl and K. Wandelt, Surface Sci. 50, 479 (1975).
M. Seo, J. B. Lumsden, and R. W. Staehle, Surface Sci. 50, 541 (1975).
R. Michael, J. Gastaldi, C. Allasia, C. Jourdan, and J. Derren, Surface Sci. 95, 309 (1980).
J. G. Chen, J. E. Crowell, and J. T. Yates, Jr., Phys. Rev. B33, 1436 (1986).
J. Homeny and M. W. Buckley, Mater. Lett. 9, 443 (1990).
C. Argile and G. E. Rhead, Thin Film Growth Modes Monitored by Auger Electron Spectroscopy (Elsevier, New York, 1989), pp. 272-301.
G. Panzner and B. Egert, Surface Sci. 144, 651 (1984).
H. Windawi and J. R. Katzer, J. Vacuum Sci. Technol. A16, 497 (1979).
J. G. Chen, M. I. Colainni, W. H. Weinberg, and J. T. Yates, Jr., Surface Sci. 238, 13 (1990).
J. F. O'Hanlon, User's Guide to Vacuum Technology (Wiley, New York, 1989), p. 448.
R. K. Schulze, T. N. Taylor, and M. T. Paffett, J. Vacuum Sci. Technol. A12, 3054 (1994).
Bond dissociation values are taken from the Handbook of Chemistry and Physics, 74th edn., D. R. Lide, ed. (CRC Press, Boca Raton, FL, 1993), pp. 9y123-9y145.
W. Arabczyk, H.-J. Müssig, and F. Storbeck, Phys. Status Solidi A55, 437 (1979).
E. L. Hardegree, P. Ho, and J. M. White, Surface Sci. 165, 488 (1986).
L. Pauling, The Nature of the Chemical Bond, 3rd edn. (Cornell Univ. Press, Ithaca, New York, 1960), pp. 265-309.
S. Addepalli, N. Magtoto, and J. A. Kelber, Surface Sci. in press.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chen, L., Magtoto, N.P., Addepalli, S. et al. S-Induced Destabilization of Aluminum Oxide at the Fe(poly)–S–Al2O3 Interface. Oxidation of Metals 54, 285–300 (2000). https://doi.org/10.1023/A:1004602412849
Issue Date:
DOI: https://doi.org/10.1023/A:1004602412849