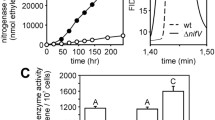
Abstract
Some rhizobial strains induce in the legume nodule a hydrogenase capable of reducing the hydrogen generated as an obligate by-product of the nitrogenase reaction. This hydrogen recycling system has a potential to improve the energy efficiency of the rhizobium-legume symbiosis. The Hup (Hydrogen uptake) system from Rhizobium leguminosarumbv. viciae UPM791 is only expressed in the legume nodule. Its genetic determinants have been isolated and characterized, and the regulation of their expression studied at the molecular level. Hydrogenase structural genes and most of the accessory genes (hup genes) are only expressed in the legume nodule under the control of NifA, the main activator of nitrogen-fixation genes. The remaining hydrogenase accessory genes (hyp genes) are expressed in microoxic vegetative cells under the control of FnrN, an Fnr-like transcriptional activator also necessary for induction of the bacteroid specific terminal oxidase. This regulatory pattern, unique to R. leguminosarum, appears to result from an adaptive evolution towards co-regulation of the hydrogenase and nitrogenase systems.
Similar content being viewed by others
References
Adams M W W, Mortenson L E and Chen J-S 1981 Hydrogenases. Biochim. Biophys. Acta 594: 105–176.
Arp D J 1992 Hydrogen cycling in symbiotic bacteria. In Biological Nitrogen Fixation. Ed. G Stacey, R H Burris and H J Evans. pp 432–460. Chapman and Hall, New York.
Black L K, Fu C and Maier J R 1994 Sequence and characterization of hupU and hupV genes of Bradyrhizobium japonicum encoding a possible nickel-sensing complex involved in hydrogenase expression. J. Bacteriol. 176: 7102–7106.
Brewin N J, DeJong T M, Phillips D A and Johnston A W R 1980 Cotransfer of determinants for hydrogenase activity and nodulation ability in Rhizobium leguminosarum. Nature 288: 77–79.
Brito B, Martínez M, Fernández D, Rey L, Cabrera E, Palacios J M, Imperial J and Ruiz-Argüeso T 1997 Hydrogenase genes from Rhizobium leguminosarum bv. viciae are controlled by the nitrogen fixation regulatory protein NifA. Proc. Natl. Acad. Sci. U.S.A. 94: 6019–6024.
Brito B, Palacios J-M, Hidalgo E, Imperial J and Ruiz-Argüeso T 1994 Nickel availability to pea (Pisum sativum L.) plants limits hydrogenase activity of Rhizobium leguminosarum bv. viciae bacteroids by affecting the processing of the hydrogenase structural subunits. J. Bacteriol. 176: 5297–5303.
Brito B, Palacios J, Imperial J, Ruiz-Argüeso T, Yang W, Bisseling T, Schmitt H, Kerl V, Bauer T, Kokotek W and Lotz W 1995 Temporal and spatial co-expression of hydrogenase and nitrogenase genes from Rhizobium leguminosarum bv viciae in pea (Pisum sativum L.) root nodules. Mol. Plant Microbe Interact. 8: 235–240.
Brito B, Palacios J-M, Ruiz-Argüeso T and Imperial J 1996 Identification of a gene for a chemoreceptor of the methyl-accepting type in the symbiotic plasmid of Rhizobium leguminosarum bv. viciae UPM791. Biochim. Biophys. Acta 1308: 7–11.
Cammack R, Fernández V M and Schneider K 1988 Nickel in hydrogenases from sulfate-reducing, photosynthetic and hydrogen oxidizing bacteria. In The Bioinorganic Chemistry of Nickel. Ed. J R Lancaster. pp 167–190. VCH Publishers, New York.
Cantrell M A, Hickok R E and Evans H J 1982 Identification and characterization of plasmids in hydrogen uptake positive and hydrogen uptake negative strains of Rhizobium japonicum. Arch. Microbiol. 131: 102–106.
Dixon R O D 1972 Hydrogenase in legume root nodule bacteroids: ocurrence and properties. Arch. Microbiol. 85: 193–201.
Dixon R O D and Wheeler C T 1986 Nitrogen fixation in plants. Blackie, Glasgow.
Eberz G and Friedrich B 1991 Three trans-acting functions control hydrogenase synthesis in Alcaligenes eutrophus. J. Bacteriol. 173: 1845–1854.
Elsen S, Colbeau A, Chabert J and Vignais P M 1996 The hupTUV operon is involved in negative control of hydrogenase synthesis in Rhodobacter capsulatus. J. Bacteriol. 178: 5174–5181.
Elsen S, Richaud P, Colbeau A and Vignais P M 1993 Sequence analysis and interposon mutagenesis of the hupT gene, which encodes a sensor protein involved in repression of hydrogenase synthesis in Rhodobacter capsulatus. J. Bacteriol. 175: 7404–7412.
Evans H J, Harker A R, Papen H, Russell S A, Hanus F J and Zuber M 1987 Physiology, biochemistry and genetics of the uptake hydrogenase in rhizobia. Annu. Rev. Microbiol. 41: 335–362.
Evans H J, Russell S A, Hanus F J, Papen H, Soto L S, Zuber M and Boursier P 1988a Hydrogenase and nitrogenase relationships in Rhizobium. Some recent developments. In Nitrogen fixation: Hundred years after. Ed. H Bothe, F J de Bruijn and WE Newton. pp 577–582. VCH, New York.
Evans H J, Russell S A, Hanus F J and Ruiz-Argüeso T 1988b The importance of hydrogen recycling in nitrogen fixation by legumes. In World crops: Cool season food legumes. Ed. R J Summerfield. pp 777–791. Kluwer Academic Publ., Boston.
Fischer H M 1994 Genetic regulation of nitrogen fixation in Rhizobia. Microbiol. Rev. 58: 352–386.
Fontecilla-Camps J C, Frey M, Garcin E, Hatchikian C, Montet Y, Piras C, Vernede X and Volbeda A 1997 Hydrogenase: A hydrogen-metabolizing enzyme. What do the crystal structures tell us about its mode of action? Biochimie 79: 661–666.
Fontecilla-Camps J C, Volbeda A and Frey M 1996 Hydrogen biocatalysis: A tale of two metals. Trends Biotechnol. 14: 417–420.
Friedrich B and Schwartz E 1993 Molecular biology of hydrogen utilization in aerobic chemolithotrophs. Annu. Rev. Microbiol. 47: 351–383.
Gutiérrez D, Hernando Y, Palacios J M, Imperial J and Ruiz-Argüeso T 1997 FnrN controls symbiotic nitrogen fixation and hydrogenase activities in Rhizobium leguminosarum bv viciae UPM791. J. Bacteriol. 179: 5264–5270.
Haugland R A, Cantrell M A, Beaty J S, Hanus F J, Russell S A and Evans H J 1984 Characterization of Rhizobium japonicum hydrogen uptake genes. J. Bacteriol. 159: 1006–1012.
Hernando Y, Palacios JM, Imperial J and Ruiz-Argüeso T 1995 The hypBFCDE operon from Rhizobium leguminosarum bv viciae is expressed from an Fnr-type promoter that escapes mutagenesis of the fnrN gene. J. Bacteriol. 177: 5661–5669.
Hernando Y, Palacios J M, Imperial J and Ruiz-Argüeso T 1998 Rhizobium leguminosarum bv. viciae hypA is expressed in pea (Pisum sativum) bacteroids and is required for hydrogenase activity and processing. FEMS Microbiol. Lett. 169: 295–302.
Hidalgo E, Leyva A and Ruiz-Argüeso T 1990 Nucleotide sequence of the hydrogenase structural genes from Rhizobium leguminosarum. Plant Mol. Biol. 15: 367–370.
Hidalgo E, Palacios J M, Murillo J and Ruiz-Argüeso T 1992 Nucleotide sequence and characterization of four additional genes of the hydrogenase structural operon from Rhizobium leguminosarum bv. viciae. J. Bacteriol. 174: 4130–4139.
Hwang J C and Burris R H 1972 Inhibition of nitrogenase-catalyzed reactions. Biochim. Biophys. Acta 283: 339–350.
Kahn D, Batut J, Daveran M-L and Fourment J 1993 Structure and regulation of the fixNOQP operon from Rhizobium meliloti. In New horizons in nitrogen fixation. Ed. R Palacios, J Mora, and W E Newton. p 474. Kluwer Academic Publishers, Dordrecht, The Netherlands.
Keyser H H, Weber D F and Uratsu S L 1984 Rhizobium japonicum serogroup and hydrogenase phenotype distribution in 12 states. Appl. Environ. Microbiol. 47: 613–615.
Kim H, Gabel C and Maier R J 1993 Expression of hydrogenase in Hupc strains of Bradyrhizobium japonicum. Arch. Microbiol. 160: 43–50.
Kim H and Maier R J 1990 Transcriptional regulation of hydrogenase synthesis by nickel in Bradyrhizobium japonicum. J. Biol. Chem. 265: 18729–18732.
Kim H, Yu C and Maier R J 1991 Common cis-acting region responsible for transcriptional regulation of Bradyrhizobium japonicum hydrogenase by nickel, oxygen and hydrogen. J. Bacteriol. 173: 3993–3999.
Lambert G R, Cantrell M A, Hanus F J, Russell S A, Haddad K R and Evans H J 1985 Intraspecies and interspecies transfer and expression of Rhizobium japonicum hydrogen uptake genes and autotrophic growth capability. Proc. Natl. Acad. Sci. U.S.A. 82: 3232–3236.
Lambert G R, Harker A R, Cantrell M A, Hanus F J, Russell S A, Haugland R A and Evans H J 1987 Symbiotic expression of cosmid-borne Bradyrhizobium japonicum hydrogenase genes. Appl. Environ. Microbiol. 53: 422–428.
Lentzsch P and Miksch G 1988 Detection of uptake hydrogenase in Rhizobium leguminosarum and Rhizobium meliloti. Zentralbl. Mikrobiol. 143: 269–274.
Lenz O and Friedrich B 1998 A novel multicomponent system mediates H2 sensing in Alcaligenes eutrophus. Proc. Natl. Acad. Sci. U.S.A. 95: 12474–12479.
Leyva A, Palacios JM, Mozo T and Ruiz-Argüeso T 1987a Cloning and characterization of hydrogen uptake genes from Rhizobium leguminosarum. J. Bacteriol. 169: 4929–4934.
Leyva A, Palacios J M and Ruiz-Argüeso T 1987b Conserved plasmid hydrogen-uptake (hup)-specific sequences within Hup+-positive Rhizobium-leguminosarum strains. Appl. Environ. Microbiol. 53: 2539–2543.
Leyva A, Palacios J M, Murillo J and Ruiz-Argüeso T 1990 Genetic organization of the hydrogen uptake (hup) cluster from Rhizobium leguminosarum. J. Bacteriol. 172: 1647–1655.
Lutz S, Jacobi A, Schlensog V, Böhm R, Sawers G and Böck A 1991 Molecular characterization of an operon (hyp) necessary for the activity of the three hydrogenase isoenzymes in Escherichia coli. Mol. Microbiol. 5: 123–135.
Maier R J and Triplett E W 1996 Toward more productive, efficient and competitive nitrogen-fixing symbiotic bacteria. Crit. Rev. Plant Sci. 15: 191–234.
Mandon K, Kaminski P A and Elmerich C 1993 Functional analysis of the fixNOQP region of Azorhizobium caulinodans. J. Bacteriol. 176: 2560–2568.
Montet Y, Amara P, Volbeda A, Vernede X, Hatchikian E C, Field M J, Frey M and Fontecilla-Camps J C 1997 Gas access to the active site of Ni-Fe hydrogenases probed by X-ray crystallography and molecular dynamics. Nature Structural Biology 4: 523–526.
O'Brian M R and Maier R J 1988 Hydrogen metabolism in Rhizobium: Energetics, regulation, enzymology and genetics. Adv. Microbial Physiol. 29: 1–52.
Palacios J M, Murillo J, Leyva A and Ruiz-Argüeso T 1990 Differential expression of hydrogen uptake (hup) genes in vegetative and symbiotic cells of Rhizobium leguminosarum. Mol. Gen. Genet. 221: 363–370.
Patschowsky T, Schlüter A and Priefer U B 1996 Rhizobium leguminosarum bv viciae contains a second fnr-fixK-like gene and an unusual fixL homologue. Mol. Microbiol. 21: 267–280.
Preisig O, Anthamatten D and Hennecke H 1993 Genes for a microaerobically induced oxidase complex in Bradyrhizobium japonicum are essential for a nitrogen-fixing endosymbiosis. Proc. Natl. Acad. Sci. U.S.A. 90: 3309–3313.
Rey L, Fernández D, Brito B, Hernando Y, Palacios J M, Imperial J and Ruiz-Argüeso T 1996 The hydrogenase gene cluster of Rhizobium leguminosarum bv. viciae contains an additional gene (hypX), which encodes a protein with sequence similarity to the N10-formyltetrahydrofolate-dependent enzyme family and is required for nickel-dependent hydrogenase processing and activity. Mol. Gen. Genet. 252: 237–248.
Rey L, Hidalgo E, Palacios J and Ruiz-Argüeso T 1992 Nucleotide sequence and organization of an H2-uptake gene cluster from Rhizobium leguminosarum bv viciae containing a rubredoxinlike gene and four additional open reading frames. J. Mol. Biol. 228: 998–1002.
Rey L, Murillo J, Hernando Y, Hidalgo E, Cabrera E, Imperial J and Ruiz-Argüeso T 1993 Molecular analysis of a microaerobically induced operon required for hydrogenase synthesis in Rhizobium leguminosarum bv. viciae. Mol. Microbiol. 8: 471–481.
Richaud P A, Colbeau B, Toussaint B and Vignais P M 1991 Identification and sequence analysis of the hupR1 gene, which encodes a response regulator of the NtrC family required for hydrogenase expression in Rhodobacter capsulatus. J. Bacteriol. 173: 5928–5932.
Rossmann R, Sauter M, Lottspeich F and Böck A 1994 Maturation of the large subunit (HYCE) of Escherichia coli hydrogenase 3 requires nickel incorporation followed by C-terminal processing at Arg537. Eur. J. Biochem. 220: 377–384.
Ruiz-Argüeso T, Hanus F J and Evans H J 1978 Hydrogen production and uptake by pea nodules as affected by strains of Rhizobium leguminosarum. Arch. Microbiol. 116: 113–118.
Sawers G 1994 The hydrogenases and formate dehydrogenases of Escherichia coli. Antonie van Leeuwenhoek 66: 57–88.
Sayavedra-Soto L A, Powell G K, Evans H J and Morris R O 1988 Nucleotide sequence of the genetic loci encoding subunits of Bradyrhizobium japonicum uptake hydrogenase. Proc. Natl. Acad. Sci. U.S.A. 85: 8395–8399.
Schlegel H G and Schneider K 1978 Distribution and physiological role of hydrogenases in microorganisms. In Hydrogenases: Their catalitic activity, structure and function. Ed. H G Schlegel and K Schneider. pp 15–44. Erich Goltze, Göttingen.
Schubert K R and Evans H J 1976 Hydrogen evolution: a major factor affecting the efficiency of nitrogen fixation in nodulated symbionts. Proc. Natl. Acad. Sci. U.S.A. 73: 1207–1211.
Schwartz E, Gerischer U and Friedrich B 1998 Transcriptional regulation of Alcaligenes eutrophus hydrogenase genes. J. Bacteriol. 180: 3197–3204.
Simpson F B and Burris R H 1984 A nitrogen pressure of 50 atmospheres does not prevent evolution of hydrogen by nitrogenase. Science 224: 1095–1097.
Stam H, van Verseveld H W and Stouthamer A H 1983 Derepression of nitrogenase in chemostat cultures of the fast growing Rhizobium leguminosarum. Arch. Microbiol. 135: 199–204.
Stam H, Stouthamer A H and van Verseveld H W 1987 Hydrogen metabolism and energy costs of nitrogen fixation. FEMS Microbiol. Rev. 46: 73–92.
Stephenson M and Stickland L H 1931 Hydrogenase: a bacterial enzyme activating molecular hydrogen I: the properties of the enzyme. Biochemistry 25: 205–214.
Thiemermann S, Dernedde J, Bernhard M, Schroeder W, Massanz C and Friedrich B 1996 Carboxyl-terminal processing of the cytoplasmic NAD-reducing hydrogenase of Alcaligenes eutrophus requires the hoxW gene product. J. Bacteriol. 178: 2368–2374.
Uratsu S L, Keyser H H, Weber D F and Lim S T 1982 Hydrogen uptake (HUP) activity of Rhizobium japonicum from major U.S. soybean areas. Crop Sci. 22: 600–602.
Van Soom C, Rumjanek N, Vanderleyden J and Neves M C P 1993a Hydrogenase in Bradyrhizobium japonicum: Genetics, regulation and effect on plant growth. World J. Microbiol. Biotechnol. 9: 615–624.
Van Soom C, Verreth C, Sampaio M J and Vanderleyden J 1993b Identification of a potential transcriptional regulator of hydrogenase activity in free-living Bradyrhizobium japonicum strains. Mol. Gen. Genet. 239: 235–240.
Vignais P M and Toussaint B 1994 Molecular biology of membranebound H2 uptake hydrogenases. Arch. Microbiol. 161: 1–10.
Volbeda A, Charon M-H, Piras C, Hatchikian E C, Frey M and Fontecilla-Camps J C 1995 Crystal structure of the nickel-iron hydrogenase from Desulfovibrio gigas. Nature 373: 580–587. Section editor: H. Lambers
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ruiz-Argüeso, T., Palacios, J.M. & Imperial, J. Regulation of the hydrogenase system in Rhizobium leguminosarum. Plant and Soil 230, 49–57 (2001). https://doi.org/10.1023/A:1004578324977
Issue Date:
DOI: https://doi.org/10.1023/A:1004578324977