Abstract
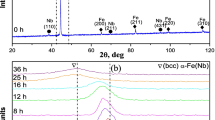
(Pb1−xBax)ZrO3 powders are synthesized below 800 °C for x ≤ 0.25 using a semi-wet route involving solid-state thermochemical reaction in a mixture of ZrO2 and (Pb1−xBax)CO3. The (Pb1−xBax)CO3 precursors were obtained by a “forced” coprecipitation technique. These powders can be sintered to achieve nearly 99% of the theoretical density at 1050 °C, which is 200 to 300 °C lower than that employed for the solid state route. The structure of as-calcined powder is orthorhombic for x ≤ 0.10 and rhombohedral for 0.25 > x ≤ 0.35, whereas the two phases coexist for 0.15 ≤ x ≤ 0.25. The structure of sintered material is orthorhombic for 0 ≤ x ≤ 0.10, rhombohedral for 0.20 ≤ x ≤ 0.30, and cubic for x ≥ 0.35, whereas orthorhombic and rhombohedral phases coexist at x = 0.15. The difference in the structure of the as-calcined and sintered powders is discussed in terms of particle size effect and chemical homogenisation of Ba2+ in the PBZ matrix.
Similar content being viewed by others
References
S. Park, M. Pan, K. Markoski, S. Yoshikawa and L. E. Cross, J. Appl. Phys. 82 (1997) 1798.
L. Goulpeau, Sov. Phys.-Solid State 8 (1967) 1970; B. A. SCOT and G. BURNS, J. Am. Ceram. Soc. 55 (1972) 331; R. W. WHATMORE and A. M. GLAZER, J. Phys. C: Solid State 12 (1979) 1505.
G. Shirane, E. Sawaguchi and Y. Takagi, Phys. Rev. 84 (1951) 476; J. HANDEREK,M. PISARSKI and Z. UJMA, J. Phys. C: Solid State 14 (1981) 2007.
G. Shirane, Phys. Rev. 86 (1952) 219.
M. K. Datta, Antiferroelectric and Ferroelectric Transitions in (Pb1¡xBax )ZrO3 System,M. Tech. Thesis in Materials Technology, Banaras Hindu University, Varanasi (INDIA) (1995).
K. H. Yoon, S. C. Hwang and D. H. Kang, J. Mat. Sc. 32 (1997) 17.
G. Shirane and S. Hoshino, Acta. Cryst. 7 (1954) 203.
E. Sawaguchi, H. Maniwa and S. Hoshino, Phys. Rev. 83 (1951) 1078.
F. Jona, G. Shirane, F. Mazzi and R. Pepinsky, ibid. 105 (1957) 849.
S. Roberts, J. Am. Ceram. Soc. 33 (1950) 63.
F. Jona, G. Shirane and R. Pepinsky, Phys. Rev. 97 (1955) 1584.
Z. Uzma, J. Handerek and M. Pisarski, Ferroelectrics, B4 (1985) 273.
V. S. Tiwari, N. Singh and D. Pandey, J. Am. Ceram. Soc. 77 (1994) 1813.
N. Singh and D. Pandey (To be published).
V. S. Tiwari, N. Singh and D. Pandey, J. Phys.: Condens. Matter, 7 (1995) 1441.
A. P. Singh, S. K. Mishra, R. Lal and D. Pandey, J. Mat. Sc. 28 (1993) 5050.
D. Pandey, A. K. Singh and A. P. Singh, Physica C 204 (1992) 179; D. PANDEY, A. K. SINGH, P. K. SHRIVASTAVA, A. P. SINGH, S. S. R. INBANATHAN and G. SINGH, Physica C 241 (1995) 279; D. PANDEY, S. S. R. INBANATHAN, P. K. SHRIVASTAVA,A. BANERJEE and G. SINGH, Physica C 261 (1996) 157.
D. Pandey, V. S. Tiwari and A. K. Singh, J. Phys. D (Appl. Phys.) 22 (1989) 182.
S. K. Mishra and D. Pandey, J. Phys. Condens. Matter 7 (1995) 9287.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pokharel, B.P., Datta, M.K. & Pandey, D. Influence of calcination and sintering temperatures on the structure of (Pb1 − xBax )ZrO3. Journal of Materials Science 34, 691–700 (1999). https://doi.org/10.1023/A:1004552308815
Issue Date:
DOI: https://doi.org/10.1023/A:1004552308815