Abstract
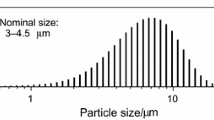
Fine powders of aluminum were produced in a pilot-plant, inert-gas atomizerwith a “confined-design” nozzle, which operated vertically upward. Argonand helium at 1.85 MPa and nitrogen at 1.56 MPa were used as the atomizingagent. The morphology of the powder particles was examined by SEM. Powderswere sieved dry and wet. The Sauter mean diameter of the powders varied from20.70 to 10.25 μm depending on the atomizing gas. The distribution ofsizes was bimodal. The mean thickness of oxide on the surface of the powderwas calculated from the total oxygen contents of powder samples (determinedby a Leco analyzer). In addition, ESCA measurements and BET tests werecarried out for surface-oxide thickness and area measurements,respectively. The finest powder produced under helium incorporated thinnersurface-oxide layers than the coarser ones produced under argon andnitrogen. This was due to differences in physical properties (such asdensity, thermal conductivity) and flow properties (such as gasvelocity and relative velocity) of the atomizing gases used, i.e., helium,argon, and nitrogen. The oxide was very irregular in thickness in thecoarse-size range of the Al powders produced under argon and nitrogen. Thiswas presumably because of the high- and low-temperature oxidation ofaluminum droplets during the atomization and subsequent solidification andcooling periods leading to the rough surfaces observed with SEMinvestigation in the present work.
Similar content being viewed by others
REFERENCES
Y. M. Kim, W. M. Griffith, and F. H. Froes, in Rapidly Solidified Al Alloys, ASTM STP 890, M. E. Fine and E. A. Starke Jr., eds. (ASTM, Philadelphia, PA, 1986), pp. 283-303.
K. Wefers and G. M. Bell, Tech. Paper No. 19, Alcoa Research Labs, Alcoa Center, PA, 1972.
J. W. Bohlen, R. J. Kar, and G. R. Chanani, in Rapidly Solidified Powder Al Alloys, ASTM STP 890, M. E. Fine and E. A. Starke Jr., eds. (ASTM, Philadelphia, PA, 1986), pp. 166-185.
S. Özbilen, A. Ünal, and T. Sheppard, Int. Symp. The Physical Chemistry of Powder Metals Production and Processing (TMSySt. Mary's, Pittsburgh, PA., 1989).
A. Ünal and D. G. Robertson, Int. J. Rapid Solidification, 2, 219 (1986).
A. Ünal, Mater. Sci. Technol. 3, 1029 (1987).
S. Özbilen, A. Ünal, and T. Sheppard, Power Metall. 34, 53 (1991).
A. Ünal, Mater. Sci. Technol. 5, 1027 (1989).
A. Ünal, Int. J. Powder Metall. Tech. 26, 11 (1990).
L. Nyborg, A. Nyland, and I. Olefjord, Surface and Interface Anal. 12, 110 (1988).
A. Ünal, Metall. Trans. 20B, 61 (1989).
A. Ünal, Mater. Sci. Technol. 4, 905 (1989).
A. Ünal, Aluminum Technology 86, Conf. Proc., T. Sheppard, ed. (Institute Metals, London, 1986), pp. 673-678.
H. Lubanska, J. Metall. 22, 45 (1970).
I. Olefjord and A. Karlsson, Aluminum Technology 86, Conf. Proc., T. Sheppard, ed. (Institute of Metals, London, 1986), pp. 383-387.
I. Olefjord, H. J. Mathiea, and P. Marcus, Corros. Sci. 3, 45 (1994).
P. Kofstad, High Temperature Oxidation of Metals (Wiley, New York, 1966), pp. 22-24.
C. J. J. Booker, Corrosion (MetalyEnvironment Reactions), L. L. Shreir, ed. (Newnes-Butterworths, London, 1977), p. 1.
J. Benard, ASM Book, p. 1 (1971).
H. H. Uhlig, Corros. Sci. 7, 325 (1976).
J. Benard, Acta Metall. 8, 272 (1960).
S. Özbilen, Powder Metall., 42, 70 (1999).
W. W. Harris, F. L. Ball, and A. T. Gwathmey, Acta Metall. 5, 574 (1957).
J. Benard, F. Gronlund, J. Oudar, and M. Duret, Zelektrochem. 63, 799 (1959).
J. Paidassi, Acta Metall. 6, 562 (1958).
J. Moreau and J. Benard, J. Chim. Phys. p. 787 (1956).
A. F. Beck, M. A. Heine, E. J. Kaule, and M. J. Pryor, Corros. Sci. 7, (1967).
M. W. Maltsev, Yu. D. Chistyakov, and M. I. Tsypin, C. R. Acad. Sci. 99, 813 (1954).
M. Chathcart, ASM Book, p. 17 (1971).
N. F. Mott, Oxid. Met., p. 59 (1970).
R. Hales, Corros. Sci. 12, 555 (1972).
J. E. Castle, Corrosion (MetalyEnvironment Reactions), L. L. Shreir, ed. (Newnes-Butterworth, London, 1977), p. 1.
P. J. Harrop, J. Mater. Sci. 3, 206 (1968).
B. C. Masters and J. E. Harris, Proc. Roy. Soc. 292A, 240 (1966).
W. E. Boggs, R. H. Kachik, and G. E. Pellissier, J. Electrochem. Soc., 112, 539 (1965).
M. E. Taylor, E. Holmes, and P. J. Boden: Corros. Sci. 9, 683 (1969).
A. J. Pignicco and G. E. Pellissier, J. Electrochem. Soc. 112, 1188 (1965).
E. A. Gulbransen, Ind. Eng. Chem. 41, 1385 (1949).
A. T. Gwayhmey and K. R. Lawless, in The Surface Chemistry of Metals and Semiconductors (Wiley, New York, 1960), p. 483.
J. V. Loukonis and R. V. Coleman, J. Appl. Phys. 30, 1364 (1969).
K. Thomas and M. W. Roberts, J. Appl. Phys. 32, 70 (1961).
A. Steinheil, Ann. Phys. 5, 465 (1934).
A. T. Gwathmey and K. R. Lawless, Acta Metall. 4, 153 (1965).
N. F. Mott, Oxidation of Metals and Alloys (ASM, Materials Park, OH, 1971), p. 37.
K. Thomas and M. W. Roberts, J. Appl. Phys. 32, 70 (1961).
D. W. Aylmore, S. H. Gregg, and W. P. Jepson, J. Inst. Metall. 88, 205 (1960).
M. S. Hunter and P. Powle, J. Electrochem. Soc. 1103, 482 (1956).
C. N. Cochran and W. C. Sleppy, J. Electrochem. Soc. 108, 322 (1961).
O. Kubascewski, Z. Elektrochem. 63, 823 (1959).
N. G. Schmal, H. Baumann, and H. Schneck, Arch. Eisenhuttenw, 27, 707 (1967).
O. Kubascewski and B. E. Hopkins, Oxidation of Metals and Alloys, 2nd edn. (Butterworth, London, 1967).
E. A. Gulbransen and W. S. Wysong, J. Appl. Chem. 51, 108 (1947).
W. W. Smeltzer, J. Electrochem. Soc. 103, 209 (1956).
E. A. Gulbransen and W. S. Wysong, J. Phys. Colloid. Chem. 57, 1087 (1947).
T. B. Grimley, Corrosion (MetalyEnvironment Reactions), L. L. Shreir, ed. (Newnes-Butterworth, London, 1977), p. 1.
N. Cabrera and N. F. Mott, Rep. Prog. Phys. 12, 163 (1948/49).
N. Cabrera and J. Hammon, Rep. Prog. Phys. 5, 47 (1947).
G. Hass, Z. Anong. Chem. 254, 96 (1947).
G. D. Preston and L. L. Bircumsham, Phil. Mag. 22, 654 (1936).
L. De Brouckere, J. Inst. Met. 71, 131 (1945).
F. Keller and J. D. Edwards, Int. Conf. Surface Reactions (Pittsburgh, PA, 1948), p. 202.
H. C. R. Raetler, Acad. Sci. Paris, 227, 124 (1948).
H. G. F. Wildsrof, Nature 168, 600 (1951).
J. A. Davies, B. Domeij, J. P. S. Pringle, and F. Brown, J. Electrochem. Soc. 112, 675 (1965).
A. Dekker and A. Middelhoek, J. Electrochem. Soc. 117, 440 (1960).
A. Guntherschulze and H. Betz, Z. Phys. 71, 106 (1932).
J. Herenguel and J. Boghen, Rev. Metall. Paris 51, 265 (1954).
G. Hass, Optik 1, 134 (1946).
M. J. Dignam, J. Electrochem. Soc. 109, 184 (1962).
M. J. Digham, J. Electrochem. Soc. 109, 192 (1962).
P. E. Doherty and R. S. Davis, J. Appl. Phys. 34, 619 (1963).
J. J. Randall and W. J. Benard, J. Appl. Phys. 35, 1317 (1964).
W. D. Treadwell and A. Obrist, Helv. Chim. Acta 26, 1816 (1943).
I. Olefjord and L. Nyborg, Powder Metall. 28, 237 (1985).
I. Olefjord and L. Nyborg, Powder Metall. 31, 33 (1988).
L. S. Darken and R. S. Gurry, Physical Chemistry of Metals (McGraw-Hill, New York, 1953), p. 349.
A. Nylund and I. Olefjord, Mater. Sci. Eng. A134, 1225 (1991).