Abstract
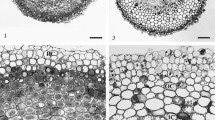
The paper investigates how the apoplastic route of ion transfer is affected by the outermost cortex cell layers of a primary root. Staining of hand-made cross sections with aniline blue in combination with berberine sulfate demonstrated the presence of casparian bands in the endo- and exodermis, potentially being responsible for hindering apoplastic ion movement. The use of the apoplastic dye Evan's Blue allowed viewing under a light microscope of potential sites of uncontrolled solute entry into the apoplast of the root cortex which mainly consisted of injured rhizodermis and/or exodermis cells. The distribution of the dye after staining was highly comparable to EDX analyses on freeze-dried cryosectioned roots. Here, we used Rb+ as a tracer for K+ in a short-time application on selected regions of intact roots from intact plants. After subsequent quench-freezing with liquid propane the distribution of K+ and Rb+ in cell walls was detected on freeze-dried cryosections by their specific X-rays resulting from the incident electrons in a SEM. All such attempts led to a single conclusion, namely, that the walls of the two outermost living cell sheaths of the cortex largely restrict passive solute movements into the apoplast. The ring of turgescent living rhizodermis cells in the root tip region forms the first barrier. With increasing distance to the root tip, in the course of their maturation resp. degradation, this particular function of the rhizodermis cells is replaced by the hypodermis resp. exodermis. Furthermore, the restriction of apoplastic ion flow by the outermost cortex cell layers is rather effective but not complete. Thus, the solute transfer into the stele is mainly restricted by the casparian bands of the endodermis. The overall conclusion is that the resistances of the rhizodermis and exodermis are additive to the endodermis in their role of regulating the apoplastic solute movement across roots.
Similar content being viewed by others
References
Aikman D P, Harmer R and Rust T S O 1980 Electrical resistance and ion movement through excised discs of sugar beet root tissue. Physiol. Plant. 48, 395-402.
Bayliss C, Van der Weele C and Canny M J 1996 Determinations of dye diffusivities in the cell-wall apoplast of roots by a rapid method. New Phytol. 134, 1-4.
Brundrett M C, Enstone D E and Peterson C A 1988 A berberine-aniline blue staining procedure for suberin, lignin, and callose in plant tissue. Protoplasma 146, 133-142.
Clarkson D T 1996 Root structure and sites of ion uptake. In Plant roots. The hidden half. 2nd edition, revised and expanded. Eds. Waisel Y, Eshel A and Kafkafi U. pp. 483-503. M Dekker, New York, Basel, Hong Kong.
Cruz R T, Jordan W R and Drew M C 1992 Structural changes and associated reduction of hydraulic conductance in roots of Sorghum bicolor L. following exposure to water deficit. Plant Physiol. 99, 203-212.
Damus M, Peterson R L, Enstone D E and Peterson C A 1997 Modification of cortical cell walls in roots of seedless vascular plants. Bot. Acta 110, 190-195.
Echlin P 1992 Low Temperature Microscopy and Analysis. Plenum Press, New York, London. 539 p.
Enstone D E and Peterson C A 1992 The apoplastic permeability of corn root apices. Can. J. Bot. 70, 1502-1512.
Esau, K 1969 Pflanzenanatomie. Gustav Fischer Verlag, Stuttgart. pp. 353-395.
Frey B, Brunner I, Walther P, Scheidegger C and Zierold K 1997 Element localisation in ultrathin cryosections of high pressure frozen ectomycorrhizal roots. Plant Cell Environ. 20, 929-937.
Kochian L V and Lucas W J 1983 Potassium transport in corn roots. II. The significance of the root periphery. Plant Physiol. 73, 208-215.
Leigh R A and Wyn Jones R G 1984 A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytol. 97, 1-13.
Marschner H 1995 Mineral nutrition of higher plants. Academic Press, London, San Diego, New York, Boston, Sydney, Toronto. pp. 537-595.
McCully M E and Boyer J S 1997 The expansion of maize root-cap mucilage during hydration. 3. Changes in water potential and water content. Physiol. Plant. 99, 169-177.
Moon G J, Peterson C A and Peterson R L 1984 Structural, chemical and permeability changes following wounding in onion roots. Can. J. Bot. 62, 2253-2259.
Newbury D E, Joy D C, Echlin P, Fiori C E and Goldstein J I 1986 Advanced Scanning Electron Microscopy and X-Ray Microanalysis. Plenum Press, New York and London. 454 p.
Perumalla C J, Peterson C A and Enstone D E 1990 A survey of angiosperm species to detect hypodermal casparian bands. I. Roots with uniseriate hypodermis and epidermis. Bot. J. Linn. Soc. 103, 93-112.
Peterson C A 1987 The exodermal casparian band of onion roots blocks the apoplastic movement of sulfate ions. J. Exp. Bot. 38, 2068-2081.
Peterson C A 1988 Exodermal casparian bands: their significance for ion uptake by roots. Physiol. Plant. 72, 204-208.
Peterson C A 1989 Significance of the exodermis in root function. In: Structural and functional aspects of transport in roots. Ed. B.C. Loughman, O. Gašpariková and J. Kolek. pp. 35-40. Kluwer Academic Publishers, Dordrecht.
Peterson C A and Emanuel M E 1983 casparian bands occur in onion root hypodermal cells: evidence from band plasmolysis. Ann. Bot. 51, 135-137.
Peterson C A, Murrmann M and Steudle E 1993 Location of the major barriers to water and ion movement in young roots of Zea mays L. Planta 190, 127-136.
Peterson C A and Perumalla C J 1990 A survey of angiosperm species to detect hypodermal casparian bands. II. Roots with a multiseriate hypodermis or epidermis. Bot. J. Linn. Soc. 103, 113-125.
Peterson C A, Peterson R L and Robards A W 1978 A correlated histochemical and ultrastructural study of the epidermis and hypodermis of onion roots. Protoplasma 96, 1-21.
Robards A W, Clarkson D T and Sanderson J 1979 Structure and permeability of the epidermal/hypodermal layers of the sand sedge (Carex arenaria, L.). Protoplasma 101, 331-347.
Shone M G T and Clarkson D T 1988 Rectification of radial water flow in the hypodermis of nodal roots of Zea mays. Plant and Soil 111, 223-229.
Steudle E 1989 Water flow in plants and its coupling to other processes: an overview. Methods in Enzymology 174, 183-225.
Steudle E and Frensch J 1996 Water transport in plants: role of the apoplast. Plant and Soil 187, 67-79.
Steudle E, Murrmann M and Peterson C A 1993 Transport of water and solutes across maize roots modified by puncturing the endodermis. Plant Physiol. 103, 335-349.
Taylor J and West D W 1980 The use of Evan's Blue stain to test the survival of plant cells after exposure to high salt and high osmotic pressure. J. Exp. Bot. 31, 571-576.
Zierold K 1986 Preparation of cryosections for biological microanalysis. In: The Science of Biological Specimen Preparation 1985. Eds. Müller M, Becker R P, Boyde A and J J Wolosewick pp. 119-127. SEM Inc., AMF O'Hare (Chicago), IL60666-0507, USA.
Zierold K 1988 X-ray microanalysis of freeze-dried and frozen-hydrated cryosections. J. Electr. Microsc. Techn. 9, 65-82.
Zimmermann H M and Steudle E 1998 Apoplastic transport across young maize roots: effect of the exodermis. Planta 206, 7-19.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gierth, M., Stelzer, R. & Lehmann, H. An analytical microscopical study on the role of the exodermis in apoplastic Rb+(K+) transport in barley roots. Plant Soil 207, 209–218 (1999). https://doi.org/10.1023/A:1004437516331
Issue Date:
DOI: https://doi.org/10.1023/A:1004437516331