Abstract
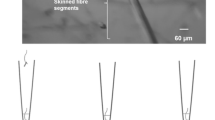
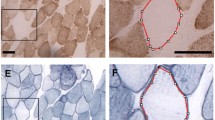
The subcellular appearance of NADPH diaphorase activity in different rat skeletal muscles has been analyzed. Both a sarcolemma-associated as well as a non-sarcolemma-associated NADPH diaphorase-dependent generation of formazan was observed. The sarcolemma-associated NADPH diaphorase staining appeared regularly in two manifestations: one observed in longitudinal sections as dotted costameres at the cell surface which accordingly appeared in transversal sections as rings surrounding the myofibre surface. At this site, nitric oxide synthase (NOS)-1 was located. The second sarcolemma-associated site of NADPH diaphorase staining was found as bundles of longitudinal-orientated stripes of hitherto unidentified origin. The non-sarcolemma-associated production of formazan was likewise manifested at two sites: the first was found regularly in longitudinal sections as intense sarcomere-like striations occurring parallel to the I-bands and indicating mitochondria. The second non-sarcolemma-associated NADPH diaphorase staining was realized as fine longitudinal filaments of variable occurrence connecting the mitochondria and presumably belonging to the sarcoplasmic reticulum. Attempts to identify single NADPH diaphorase(s) existing in skeletal muscles by incubation with specific inhibitors failed but showed the presence of two different subpopulations of NADPH diaphorases in myofibres: a urea-resistant fraction in the sarcolemma region containing NOS-1 and a non-sarcolemma-associated, urea-sensitive fraction depleted of NOS-1.
Similar content being viewed by others
References cited
Blottner D, Grozdanovic Z, Gossrau R (1995) Histochemistry of nitric oxide synthase in the nervous system. Histochem J 27: 785–811.
Bredt DS, Hwang PM, Glatt CE, Lowenste C, Reed RR, Snyder SH (1991) Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature 351: 714–718.
Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS (1996) Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell 84: 757–767.
Chang WJ, Iannaccone ST, Lau KS, Masters BS, McCabe TJ, McMillan K, Padre RC, Spencer MJ, Tidball JG, Stull JT (1996) Neuronal nitric oxide synthase and dystrophin-deficient muscular dystrophy. Proc Natl Acad Sci USA 93: 9142–9147.
Cooper MB, Craft JA, Estall MR, Rabin BR (1980) Asymmetric distribution of cytochrome P-450 and NADPH-cytochrome P-450 (cytochrome c) reductase in vesicles from smooth endoplasmic reticulum of rat liver. Biochem J 190: 737–746.
Fahimi HD, Amarasingham CR (1964) Cytochemical localization of lactic dehydrogenase in white skeletal muscle. J Cell Biol 22: 29–48.
Fahimi HD, Karnovsky MJ (1966) Cytochemical localization of two glycolytic dehydrogenases in white skeletal muscle. J Cell Biol 29: 113–128.
Förstermann U, Gath I, Schwarz P, Closs EI, Kleinert H (1995) Isoforms of nitric oxide synthase. Properties, cellular distribution and expressional control. Biochem Pharmacol 50: 1321–1332.
Frandsen U, Lopez-Figueroa M, Hellsten Y (1996) Localization of nitric oxide synthase in human skeletal muscle. Biochem Biophys Res Commun 227: 88–93.
Gath I, Ebert J, Godtel-Armbrust U, Ross R, Reske-Kunz AB, Förstermann U (1999) NO synthase II in mouse skeletal muscle is associated with caveolin 3. Biochem J 340: 723–728.
Gossrau R (1998a) Caveolin-3 and nitric oxide synthase I in healthy and diseased skeletal muscle. Acta Histochem 100: 99–112.
Gossrau R (1998b) Nitric oxide synthase I (NOS I) is a costameric enzyme in rat skeletal muscle. Acta Histochem 100: 451–462.
Grozdanovic Z, Gossrau R (1995) Alpha-NADPHappears to be primarily oxidized by the NADPH-diaphorase activity of nitric oxide synthase (NOS). Acta Histochem 97: 313–320.
Grozdanovic Z, Nakos G, Dahrmann G, Mayer B, Gossrau R (1995) Species-independent expression of nitric oxide synthase in the sarcolemma region of visceral and somatic striated muscle fibres. Cell Tissue Res 281: 493–499.
Haramaki N, Han D, Handelman GJ, Tritschler HJ, Packer L (1997) Cytosolic and mitochondrial systems for NADH-and NADPH-dependent reduction of alpha-lipoic acid. Free Radic Biol Med 22: 535–542.
Hope BT, Michael GJ, Knigge KM, Vincent SR (1991) Neuronal NADPH diaphorase is a nitric oxide synthase. Proc Natl Acad Sci USA 88: 2811–2814.
Jaiswal AK (1994) Human NAD(P)H: quinone oxidoreductase 2. Gene structure, activity, and tissue-specific expression. J Biol Chem 269: 14502–14508.
Kobzik L, Stringer B, Balligand JL, Reid MB, Stamler JS (1995) Endothelial type nitric oxide synthase in skeletal muscle fibres: mitochondrial relationships. Biochem Biophys Res Commun 211: 375–381.
Komuro A, Tobe T, Hashimoto K, Nakano Y, Yamaguchi T, Nakajima H, Tomita M (1996) Molecular cloning and expression of human liver biliverdin-IX beta reductase. Biol Pharm Bull 19: 796–804.
Kulkoski JA, Weber JL, Ghazarian JG (1979) NADPH-cytochrome c reductase in outer membrane of kidney mitochondria. Purification and properties. Arch Biochem Biophys 192: 539–547.
Kusner LL, Kaminski HJ (1996) Nitric oxide synthase is concentrated at the skeletal muscle endplate. Brain Res 730: 238–242.
Lind C, Cadenas E, Hochstein P, Ernster L (1990) DT-diaphorase: purification, properties, and function. Methods Enzymol 186: 287–301.
Lojda Z, Gossrau R, Schiebler TH (1979) Enzyme Histochemistry. A Laboratory Manual. Berlin, Heidelberg, New York: Springer.
Massey V, Schopfer LM (1986) Reactivity of old yellow enzyme with alpha-NADPH and other pyridine nucleotide derivatives. J Biol Chem 261: 1215–1222.
Nakos G, Gossrau R (1994) When NADPH diaphorase (NADPHd)works in the presence of formaldehyde, the enzyme appears to visualize selectively cells with constitutive nitric oxide synthase (NOS). Acta Histochem 96: 335–343.
Nishida CR, Ortiz de Montellano PR (1998) Electron transfer and catalytic activity of nitric oxide synthases. Chimeric constructs of the neuronal, inducible, and endothelial isoforms. J Biol Chem 273: 5566–5571.
Olausson T, Fjellstrom O, Meuller J, Rydstrom J (1995) Molecular biology of nicotinamide nucleotide transhydrogenase-a unique proton pump. Biochim Biophys Acta 1231: 1–19.
Ortiz de Montellano PR, Nishida C, Rodriguez-Crespo I, Gerber N (1998) Nitric oxide synthase structure and electron transfer. Drug Metab Dispos 26: 1185–1189.
Scherer-Singler U, Vincent SR, Kimura H, McGeer EG (1983) Demonstration of a unique population of neurons with NADPH-diaphorase histochemistry. J Neurosci Meth 9: 229–234.
Schuelke M, Gossrau R, Huang R, Fishman M (1998) Histochemical studies of skeletal muscles in NOS I knockout mice. Ann Anat 181(Suppl.): 311.
Shalloe F, Elliott G, Ennis O, Mantle TJ (1996) Evidence that biliverdin-IX beta reductase and flavin reductase are identical. Biochem J 316: 385–387.
Stoward PJ (1991) Dehydrogenases. In: Stoward PJ, Pearse AGE, eds. Histochemistry: Theoretical and Applied. Vol. 3, Edinburgh: Livingstone, pp. 27–71.
Tinsley JM, Blake DJ, Davies KE (1997) Localization of dystrophin in skeletal, cardiac and smooth muscle. In: Brown SC, Lucy JA, eds. Dystrophin: Gene, Protein and Cell Biology. Vol. 1, Cambridge, New York, Melbourne: Cambridge University Press, pp. 56–78.
Watkins SC, Swartz DR, Byers TJ (1997) Localization of dystrophin in skeletal, cardiac and smooth muscle. In: Brown SC, Lucy JA, eds. Dystrophin: Gene, Protein and Cell Biology. Vol. 1, Cambridge, New York, Melbourne: Cambridge University Press, pp. 79–104.
Young HM, O'Brien AJ, Furness JB, Ciampoli D, Hardwick JP, McCabe TJ, Narayanasami R, Masters BS, Tracey WR (1997) Relationships between NADPH diaphorase staining and neuronal, endothelial, and inducible nitric oxide synthase and cytochrome P450 reductase immunoreactivities in guinea-pig tissues. Histochem Cell Biol 107: 19–29.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Planitzer, G., Baum, O. & Gossrau, R. Skeletal Muscle Fibres Show NADPH Diaphorase Activity Associated with Mitochondria, the Sarcoplasmic Reticulum and the NOS-1-containing Sarcolemma. Histochem J 32, 303–312 (2000). https://doi.org/10.1023/A:1004041129915
Issue Date:
DOI: https://doi.org/10.1023/A:1004041129915