Abstract
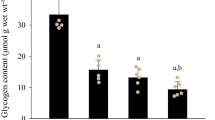
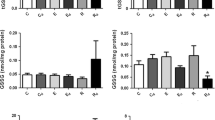
Glycogenin, glycogen-debranching enzyme (GDE) and glycogen phosphorylase (GP) are important enzymes that contribute to glycogen particle metabolism. In Long-Evans Hooded rat whole muscle homogenates prepared from extensor digitorum longus (EDL, fast-twitch) and soleus (SOL, oxidative, predominantly slow twitch), it was necessary to include α-amylase, which releases glucosyl units from glycogen, to detect glycogenin but not GDE or GP. Up to ∼12 % of intramuscular glycogen pool was broken down using either in vitro electrical stimulation or leaving muscle at room temperature >3 h (delayed, post-mortem). Electrical stimulation did not reveal glycogenin unless α-amylase was added, although in post-mortem muscle ∼50 and ∼30 % of glycogenin in EDL and SOL muscles, respectively, was detected compared to the amount detected with α-amylase treatment. Single muscle fibres were dissected from fresh or post-mortem EDL muscles, mechanically skinned to remove surface membrane and the presence of glycogenin, GDE and GP as freely diffusible proteins (i.e. cytoplasmic localization) compared by Western blotting. Diffusibility of glycogenin (∼20 %) and GP (∼60 %) was not different between muscles, although GDE increased from ∼15 % diffusible in fresh muscle to ∼60 % in post-mortem muscle. Under physiologically relevant circumstances, in rat muscle and within detection limits: (1) The total cellular pool of glycogenin is always associated with glycogen granules, (2) GDE is associated with glycogen granules with over half the total pool associated with the outer tiers of glycogen, (3) GP is only ever weakly associated with glycogen granules and (4) addition of α-amylase is necessary in order to detect glycogenin, but not GDE or GP.







Similar content being viewed by others
References
Baque S, Guinovart JJ, Ferrer JC (1997) Glycogenin, the primer of glycogen synthesis, binds to actin. FEBS Lett 417:355–359
Campbell DG, Cohen P (1989) The amino acid sequence of rabbit skeletal muscle glycogenin. Eur J Biochem 185:119–125
Essen B (1978) Glycogen depletion of different fibre types in human skeletal muscle during intermittent and continuous exercise. Acta Physiol Scand 103:446–455
Goodman C, Blazev R, Stephenson G (2005) Glycogen content and contractile responsiveness to T-system depolarization in skinned muscle fibres of the rat. Clin Exp Pharmacol Physiol 32:749–756
Graham TE, Yuan Z, Hill AK, Wilson RJ (2010) The regulation of muscle glycogen: the granule and its proteins. Acta Physiol Oxford 199:489–498
Greenhaff PL, Soderlund K, Ren JM, Hultman E (1993) Energy metabolism in single human muscle fibres during intermittent contraction with occluded circulation. J Physiol 460:443–453
Hansen BF, Derave W, Jensen P, Richter EA (2000) No limiting role for glycogenin in determining maximal attainable glycogen levels in rat skeletal muscle. Am J Physiol Endocrinol Metab 278:E398–E404
Kraniou Y, Cameron-Smith D, Misso M, Collier G, Hargreaves M (2000) Effects of exercise on GLUT-4 and glycogenin gene expression in human skeletal muscle. J Appl Physiol 88:794–796
Lau X, Zhang Y, Kelly DJ, Stapleton DI (2013) Attenuation of Armanni-Ebstein lesions in a rat model of diabetes by a new anti-fibrotic, anti-inflammatory agent, FT011. Diabetologia 56:675–679
Lomako J, Lomako WM, Whelan WJ (1988) A self-glucosylating protein is the primer for rabbit muscle glycogen biosynthesis. FASEB Journal 2:3097–3103
Manasek FJ (1969) Myocardial cell death in the embryonic chick ventricle. J Embryol Exp Morphol 21:271–284
Marchand I, Chorneyko K, Tarnopolsky M, Hamilton S, Shearer J, Potvin J, Graham TE (2002) Quantification of subcellular glycogen in resting human muscle: granule size, number, and location. J Appl Physiol 93:1598–1607
Melendez R, Melendez-Hevia E, Cascante M (1997) How did glycogen structure evolve to satisfy the requirement for rapid mobilization of glucose? A problem of physical constraints in structure building. J Mol Evol 45:446–455
Mollica JP, Oakhill JS, Lamb GD, Murphy RM (2009) Are genuine changes in protein expression being overlooked? Reassessing Western blotting. Anal Biochem 386:270–275
Murphy RM, Lamb GD (2013) Important considerations for protein analyses using antibody based techniques: down-sizing Western blotting up-sizes outcomes. J Physiol Lond 591:5823–5831
Murphy RM, Mollica JP, Beard NA, Knollmann BC, Lamb GD (2011) Quantification of calsequestrin 2 (CSQ2) in sheep cardiac muscle and Ca2 + −binding protein changes in CSQ2 knockout mice. Am J Physiol Heart Circ Physiol 300:H595–H604
Murphy RM, Mollica JP, Lamb GD (2009) Plasma membrane removal in rat skeletal muscle fibers reveals caveolin-3 hot-spots at the necks of transverse tubules. Exp Cell Res 315:1015–1028
Murphy RM, Verburg E, Lamb GD (2006) Ca2+ activation of diffusible and bound pools of mu-calpain in rat skeletal muscle. J Physiol 576:595–612
Murphy RM, Xu H, Latchman H, Larkins NT, Gooley PR, Stapleton DI (2012) Single fiber analyses of glycogen-related proteins reveal their differential association with glycogen in rat skeletal muscle. Am J Physiol Cell Physiol 303:C1146–C1155
Nielsen J, Schroder HD, Rix CG, Ortenblad N (2009) Distinct effects of subcellular glycogen localization on tetanic relaxation time and endurance in mechanically skinned rat skeletal muscle fibres. J Physiol 587:3679–3690
Park KH, Kim TJ, Cheong TK, Kim JW, Oh BH, Svensson B (2000) Structure, specificity and function of cyclomaltodextrinase, a multispecific enzyme of the alpha-amylase family. Biochim Biophys Acta 1478:165–185
Parker GJ, Koay A, Gilbert-Wilson R, Waddington LJ, Stapleton D (2007) AMP-activated protein kinase does not associate with glycogen alpha-particles from rat liver. Biochem Biophys Res Commun 362:811–815
Patterson MF, Stephenson GM, Stephenson DG (2006) Denervation produces different single fiber phenotypes in fast- and slow-twitch hindlimb muscles of the rat. Am J Physiol Cell Physiol 291:C518–C528
Pitcher J, Smythe C, Campbell DG, Cohen P (1987) Identification of the 38-kDa subunit of rabbit skeletal muscle glycogen synthase as glycogenin. Eur J Biochem 169:497–502
Pitcher J, Smythe C, Cohen P (1988) Glycogenin is the priming glucosyltransferase required for the initiation of glycogen biogenesis in rabbit skeletal muscle. Eur J Biochem 176:391–395
Ryu JH, Drain J, Kim JH, McGee S, Gray-Weale A, Waddington L, Parker GJ, Hargreaves M, Yoo SH, Stapleton D (2009) Comparative structural analyses of purified glycogen particles from rat liver, human skeletal muscle and commercial preparations. Int J Biol Macromol 45:478–482
Shearer J, Graham TE, Battram DS, Robinson DL, Richter EA, Wilson RJ, Bakovic M (2005) Glycogenin activity and mRNA expression in response to volitional exhaustion in human skeletal muscle. J Appl Physiol 99:957–962
Smythe C, Watt P, Cohen P (1990) Further studies on the role of glycogenin in glycogen biosynthesis. Eur J Biochem 189:199–204
Taylor C, Cox AJ, Kernohan JC, Cohen P (1975) Debranching enzyme from rabbit skeletal muscle. Purification, properties and physiological role. Eur J Biochem 51:105–115
Tsintzas OK, Williams C, Boobis L, Greenhaff P (1995) Carbohydrate ingestion and glycogen utilization in different muscle fibre types in man. J Physiol 489(Pt 1):243–250
Zierath JR, Hawley JA (2004) Skeletal muscle fiber type: influence on contractile and metabolic properties. PLoS Biol 2:e348
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, H., Stapleton, D. & Murphy, R.M. Rat skeletal muscle glycogen degradation pathways reveal differential association of glycogen-related proteins with glycogen granules. J Physiol Biochem 71, 267–280 (2015). https://doi.org/10.1007/s13105-015-0407-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-015-0407-y