Abstract
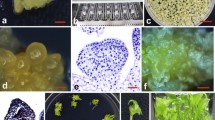
The successful application of plant biotechnology to Alstroemeria improvement will largely depend on the availability of an efficient regeneration/transformation system. Regeneration in Alstroemeria is accomplished from nodular embryogenic callus initiated from zygotic embryos. Histological studies of embryogenic callus initiation from 4-weeks old cultured ovules revealed that the outermost layers of the protoderm of the embryogenic nodules divided to form either a new nodule or aproembryo. Transient gene expression after particle bombardment of nodular embryogenic callus was optimized using DNA of pAHC25. The highest β-glucuronidase expression was found when the GUS gene was under control of the maize ubiquitin promoter, the target tissue was placed 5 cm below the microcarrier launch assembly and when the rupture disc-breakage point was between 650–900 psi. Kanamycin blocked regeneration of somatic embryos, however, did not block growth of nodular embryogenic callus. With phosphinothricin both callus growth and regeneration were blocked. Bombardment of nodular embryogenic callus with DNA of pAHC25 combined with selection on medium containing phosphinothricin resulted in putative transgenic chimeric. Friable calli were selected from nodular embryogenic callus and used to initiate suspensions. These cell suspensions were subjected to transformation by particle bombardment using DNA of pAHC25 and resulted in a stable transformed friable callus line after selection based on luciferase activity. Even after 2 years of maintenance this callus line was luciferase positive and the Polymerase Chain Reaction analysis demonstrated the presence of the introduced gene in this friable callus line.
Similar content being viewed by others
References
Christensen, A.H., R.A. Sharrock & P.H. Quail, 1992. Maize polyubiquitin genes, structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol Biol 18: 675-689.
Christensen, A.H. & P.H. Quail, 1996. Ubiquitin promoter-based vectors for high-levelexpression of selectable and/or screenable marker genes inmonocotyledonousplants. Trans Res 5: 213-218.
Christou, P., 1995. Strategies for variety-independent genetic transformation of important cereals, legumes and woody species utilising particle bombardment. Euphytica 85: 13-27.
Franklin, C.I., T.N. Trieu. & R.A. Gonzales, 1990. Plant regeneration through somaticembryogenesis in the forage grass Caucasian bluestem (Bothriochloa caucasica). Plant Cell Rep 9: 443-446.
Gonzalez-Benito, M.E. & P.G. Alderson, 1992. Callus induction and plant regenerationin Alstroemeria. J Exp Bot 43: 205-211.
Guerineau, F. & P.M. Mullineaux, 1993. Plant transformation and expression vectors. In: R.R.D. Croy (Ed.), Plant Molecular Biology, pp. 121-148. Labfax, BIOS Scientificpublishers, Oxford, UK.
Hutchinson, M.J., J.M. Tsujita & P.K. Saxena, 1994. Callus induction and plant regeneration from mature zygotic embryos of a tetraploid Alstroemeria (A. pelegrina × A. psittacina). Plant Cell Rep 14: 184-187.
Hutchinson, M.J., T. Senaratna, J.M. Tsujita & P.K. Saxena, 1997. Somaticembryogenesis in liquid cultures of a tetraploid Alstroemeria. Plant Cell, Tiss and Org Cult 47: 293-297.
Jefferson, R.A., T.A. Kavanagh & M.W. Bevan, 1987. Gus-fusions: β-glucuronidaseas a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901-3907.
Kikkert, J.R., 1993. The biolistic PDS-1000/He device. Plant Cell, Tiss and Org Cult 33: 221-226.
Klein, T.M., E.D. Wolf, R. Wu & J.C. Sanford, 1987. High-velocity micro-projectiles for delivering nucleic acids into living cells. Nature 327: 70-73.
Kramer, C., J. DiMaio, G.F. Carswell & R.D. Shillito, 1992. Selection of transformed protoplast-derived Zea mays colonies with phosphinothricin and novel assay using pH indicator chlorophenol red. Planta 190: 454-458.
McCabe, D.E., W.F. Swain, B.J. Martinelli, & P. Christou, 1988. Stable transformationof soybean (Glycine max) by particle acceleration. Bio/Technol 6: 923-926.
Murashige, T. & F. Skoog, 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15: 473-497.
Ow, D.W., K.V. Wood, M. DeLuca, J.R. De Wet, D.R., Helinsky & S.H. Howell, 1986. Transient and stable expression of the firefly luciferase gene in plant cells and transgenic plants. Science 234: 856-859.
Raemakers, C.J.J.M., E. Sofiari, E. Jacobsen, & R.G.F. Visser, 1997. Regeneration and transformation of cassava. Euphytica 96: 153-161.
Raemakers, C.J.J.M., E. Jacobsen, & R.G.F. Visser, 1997. Secondary somatic embryo genesis and applications for plant breeding. Euphytica 81: 93-107.
Sofiari, E., C.J.J.M. Raemakers, J.E.M. Bergervoet, E. Jacobsen & R.G.F. Visser, 1997. Plant regeneration from protoplasts isolated from friable embryogenic callus of cassava. Plant Cell Rep 18: 159-165.
Tayler, M.G. & I.K. Vasil, 1991. Histology of, and physical factors affecting, transient gus expression in pearl millet (Pennisetum glaucum (L.) R.Br.) embryosfollowing microprojectile bombardment. Plant Cell Rep 10: 120-125.
Van Schaik, C.E., A. Posthuma, M.J. de Jeu & E. Jacobsen, 1996. Plant regenerationthrough somatic embryogenesis from callus induced on immature embryos of Alstroemeria spp. L. Plant Cell Rep 15: 377-380.
Velten, J., Velten L., Hain, R. & J. schell, 1984. Isolation of a dual promoter fragment from the ti plasmid of Agrobacterium tumefaciens. EMBO J 3: 2723-2730.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van Schaik, C., van der Toorn, C., De Jeu, M. et al. Towards genetic transformation in the monocot Alstroemeria L.. Euphytica 115, 17–26 (2000). https://doi.org/10.1023/A:1003979423469
Issue Date:
DOI: https://doi.org/10.1023/A:1003979423469