Abstract
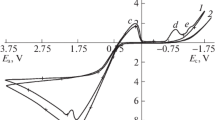
The stripping and electrodeposition of silver in fluorosilicic acid solution are irreversible reactions as indicated by cyclic voltammetry. The rate constant for the intrinsic heterogeneous cathodic deposition of silver was obtained as k 0C =40.77 exp(1.88−29.43 E 0′C ) and the diffusion coefficient of the silver ion in fluorosilicic acid solution as 5.17×10−5 cm2 s−1. Theoretical calculations of the concentration of silver ion correlated well with experimental data. The relationship between the diffusion layer thickness and the stirring rate was also obtained. Increasing the stirring rate and temperature, and decreasing the current density and concentration of fluorosilicic acid, caused an increase in the current efficiency for silver deposition on graphite.
Similar content being viewed by others
References
A.T. Kuhn, P. Neufeld and G. Butler, Surf. Technol. 16 (1982) 1.
Y. Kito, T. Sakuta, T. Mizuno and T. Morita, J. Powder Metal 25 (1989) 13.
F. Montino and L. Colombo, US Patent 4 039 317. (1977).
O.A. Short and N.J. Metochen, US Patent 2 752 237. (1956).
J.Y. Wu, Producing Fine Silver Powder, MS thesis of National Central University, Chung Li, Taiwan, ROC (1993).
E.M. Jost and P. Mass, US Patent 4 456 473. (1984).
E.M. Jost and P. Mass, US Patent 4 456 474. (1984).
T. Hayashi, European Patent 0 249 366. (1987).
M.G. Pavlovic, M.D. Maksimovic and K.I. Popov, J. Appl. Electrochem. 8 (1978) 61.
A. Calusaru, Electrodepostion of Metal Powder, Elsevier Scientific, New York (1979) pp. 345–7.
J.Y. Lee and T.C. Tan, J. Electrochem. Soc. 137 (1990) 1402.
K.I. Popov, B.A. Mitrovic, M.G. Pavlovic and B.V. Toperic, J. Appl. Electrochem. 21 (1991) 50.
E. Michailova and A. Milchev, J. Appl. Electrochem. 21 (1991) 170.
M.E. Martins, R.C. Salvarezza, J.M. Vara and A.J. Arvia, J. Electrochem. Soc. 138 (1991) 2509.
K.N. Brown and N.J. Teaneck, US Patent 2 810 682. (1957).
A.J. Bard and L.R. Faulkner, Electrochemical Methods: Fundamentals and Applications, J. Wiley & Sons, New York, (1980).
T.C. Chou, J.S. Do, B.J. Hwang and J.J. Jow, Chem. Eng. Comm. 51 (1987) 47.
R.B. Bird, W.E. Stewart and E.N. Lightfoot, Transport Phenomena, J. Wiley & Sons, New York, (1960) p. 514.
R.H. Perry and C.H. Chilton, Chemical Engineer's Handbook, 5th edn, McGraw-Hill, New York (1973) pp. 3–234.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Do, JS., Her, AS. Mass transfer and current efficiency for the electrodeposition of silver in fluorosilicic acid solution. Journal of Applied Electrochemistry 29, 827–834 (1999). https://doi.org/10.1023/A:1003646119602
Issue Date:
DOI: https://doi.org/10.1023/A:1003646119602