Abstract
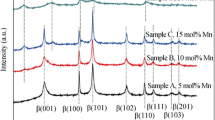
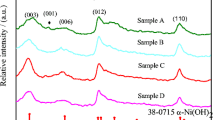
Substitution of 20 aluminium for nickel in the lattice of nickel hydroxide, prepared by coprecipitation, leads to a hydrotalcite-like compound of formula Ni0.8Al0.2(OH)2(CO3)0.1.0.66H2O. It has been found that the compound has prolonged stability in 6m KOH solution and can be used as the positive electrode material in rechargeable alkaline batteries. The structure, morphology and composition of the compound have been investigated by X-ray diffraction, scanning electron microscopy and infrared spectroscopy. The electrode comprising the aluminium-substituted nickel hydroxide has greater discharge capacity and higher utilization of active material than the β-Ni(OH)2 electrode. Cyclic voltammetry suggest that the aluminium-substituted nickel hydroxide has better reversibility of the Ni(OH)2/NiOOH redox couple and higher oxygen evolution overpotential than β-Ni(OH)2. The mechanism of the electrode reaction has also been discussed and the proton diffusion coefficient in the compound has been determined.
Similar content being viewed by others
References
A. Audemer, A. Delahaye, R. Farhi, N.S. Epee and J.M. Tarascon, J. Electrochem. Soc. 144 (1997) 2614.
A.D. Vidal and M. Figlarz, J. Appl. Electrochem. 17 (1987) 589.
R. Barnard, C.F. Randell and F.L. Tye, J. Appl. Electrochem. 10 (1980) 127.
C. Delmas, C. Faure and Y. Borthomieu, Mater. Sci. Eng. B 13 (1992) 89
C. Faure, C. Delmas and P. Willmann, J. Power Sources 36 (1991) 497.
R.D. Armstrong and E.A. Charles, J. Power Sources 25 (1989) 89.
B.B. Ezhov and O.G. Malandin, J. Electrochem. Soc. 138 (1991) 885.
C. Faure, C. Pelmas and P. Willmann, J. Power Sources. 35 (1991) 263.
L.G. Demourgues, J.J. Braconnier and C. Delmas, J. Power Sources 45 (1993) 281.
L.G. Demourgues and C. Delmas, J. Electrochem. Soc. 143 (1996) 561.
L.G. Demourgues, C. Denage and C. Delmas, J. Power Sources 52 (1994) 269.
L.G. Demourtgues and C. Delmas, J. Power Sources 52 (1994) 275.
P.V. Kamath, M. Dixit, L. India, A.K. Shukla, V.G. Kumar and N. Munichandraiah, J. Electrochem. Soc. 141 (1994) 2956.
R.D. Armstrong and H. Wang, Electrochim. Acta. 36 (1991) 759.
P. Oliva, J. Leonardi, J.F. Laurent, C. Delmas, J.J. Braconnier, M. Figlarz and F. Fievet, J. Power Sources 8 (1982) 229.
M.C. Bernard, P. Bernard, M. Keddam, S. Senyarich and H. Takenouti, Electrochim. Acta 41 (1996) 91.
L. Indira, M. Dixit and P.V. Kammath, J. Power Sources 52 (1994) 93.
H. Bode, K. Dehmelt and J. Witle, Z. Anorg. Chem. 366 (1969) 1.
P.V. Kamath and M.F. Ahmed, J. Appl. Electrochem. 23 (1993) 225.
D.A. Corrigan and R.M. Bendert, J. Electrochem. Soc. 136 (1989) 723.
X. Wang, J. Yan, H. Yuan and Y. Zhang, J. Power Sources 72 (1998) 221.
X. Wang, J. Yan, H. Yuan and Y. Zhang, J. Appl. Electrochem. in press.
C. Zhang and S.M. Park, J. Electrochem. Soc. 134 (1987) 2966.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Liu, B., Wang, X., Yuan, H. et al. Physical and electrochemical characteristics of aluminium-substituted nickel hydroxide. Journal of Applied Electrochemistry 29, 853–858 (1999). https://doi.org/10.1023/A:1003537900947
Issue Date:
DOI: https://doi.org/10.1023/A:1003537900947