Abstract
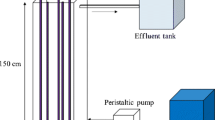
Organic compounds dissolved in water can be decomposed on a layer of n-TiO2 particles irradiated by u.v. light, which generates holes and electrons in the TiO2 material. Dissolved oxygen was used as electron scavenger and holes reacted with water to give OH radicals. The rate of degradation of the dissolved organic compounds by OH radicals is limited by the transfer of either oxygen or of theorganic compounds to the surface of n-TiO2 particles. The consequence of these limits is that, in the batch mode reactor with recirculation of the liquid, the dependence of the concentration of an organic compound on time has either a linear or an exponential form. Experiments with decomposition of oxalic acid in aqueous solutions using a plate reactor (60 cm × 120 cm) confirmed the analysis. Equations for evaluation of the mass transfer coefficient of the dissolved species to the surface of the plate reactor with a moving liquid fil m were developed for the case of the thickness of the Nernst diffusion layer being thinner than the thickness of the liquid. The experimentally obtained decomposition rate of oxalic acid was about 60 to 80% of the theoretical decomposition rate limited by oxygen flux through the film of a moving liquid. The present theory neglects the diffusion of oxygen into the porous layer of n-TiO2.
Similar content being viewed by others
References
Abstracts of the First International Conference on Advanced Oxidation Technologies for Water and Air Treatment, London, Ontario, Canada, 25–30 June (1994).
F. A. DiGiano, in ‘Control of Organic Substances in Water and Wastewater’ (edited by B. B. Berger), Noyes Data Corporation, New Jersey (1987) Chapter 3.
J. J. Rook, Water Treatment and Examination 23 (1974) 234.
T. A. Bellar, J. J. Lichtenberg and R. C. Kroner, J. American Water Works Association 66 (1974) 703.
J. J. Rook, Environ. Sci. Tech. 11 (1977) 478.
R. Rife, T. W. Thomas, D. W. Norberg, R. L. Fournier, F. G. Rinker and M. S. Bonomo, Environ. Prog. 8 (1989) 167.
R. B. Pojasek, ‘Toxic and Hazardous Waste Disposal’, Ann Arbor Science, Ann Arbor, MI (1979), Vols. I-IV.
J. B. Kim, C. S. Gee, J. T. Bandy and C. S. Huang, J. Water Pollut. Control Fed. 63 (1991) 501.
H. M. Freedman, ‘Incinerating Hazardous Wastes,’ Technomic Publishing Co., Lancaster, PA (1988), p. 375.
D. J. De Renzo, J. Biodegradation Techniques for Industrial Organic Wastes, Noyes Data Corporation, Park Ridge, NJ (1980), p. 358.
M. R. Hoffman, S. T. Martin, W. Choi, D. W. Bahneman, Chem. Rev. 95 (1995) 1.
H. Gerischer, Electrochim. Acta 38 (1993) 3.
E. Pellizeti and C. Minero, ibid. 38 (1993) 47.
D. Bockelmann, PhD. thesis, ‘Photocatalytical Solar Treatment of Waste Water’, Technical University Clausthal, Germany (1993).
R. I. Bickley, T. Gonzales-Carreno, J. S. Lees, L. Palmisano and R. J. D. Tilley, J. Sol. State Chem. 92 (1991) 178.
S. L. Wilkinson and W. A. Anderson, in Abstracts of the First International Conference, op. cit. [1], p. 299.
K. Hashimoto and A. Fujishima, in Abstracts of the First International Conference, op. cit. [1], p. 311.
J. F. Kenneke and W. H. Glaze, in Proceedings of the Symposium on Water Purification by Photocatalytic, Photoelectrochemical and Electrochemical Processes, The Electrochemical Society, 94 (1994) 365.
D. F. Ollis, E. Pelizzeti and N. Serpone, in ‘Photocatalytic Fundamentals and Application’ (edited by N. Serpone and E. Pelizzeti), Wiley, New York (1989), p. 625.
R. W. Mathews, J. Phys. Chem. 91 (1987) 3328.
C. S. Turchi and D. F. Ollis, J. Phys. Chem. 92 (1988) 6852.
R. W. Mathews, ibid. 92 (1988) 6853.
H. Brauner, ‘Grundlagen der Einphasen-und Mehrphasenstroemungen’, Verlag Sauerlaender Aarau, Frankfurt/Main (1971), p. 674.
F. Goodridge and G. Gartside, Trans. Inst. Chem. Eng. 43 (1965) 62.
I. Rousar, J. Hostomský, V. Cezner, B. {ie853–02}tverák, J. Electrochem. Soc. 118 (1971) 881.
K. Javdani, Chem. Eng. Sci. 29 (1974) 61.
J. F. Rabek, ‘Experimental Methods in Photochemistry and Photophysics’, Part II., J. Wiley & Sons, Chichester (1982), p. 947.
G. Houghton, P. D. Ritchi and J. A. Thomson, Chem. Eng. Sci. 17 (1962) 221.
Anonymous, ‘Physico-chemical Tables,’ SNTL, Prague (1953).
C. H. Pollema and J. L. Hendrix, J. Photochem. Photobiol. A: Chem. 66 (1992) 235.
J. Cunningham and Ghassam Al-Sayyed, J. Chem. Soc., Faraday Trans. 86 (1990) 3935.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kulas, J., Roušar, I., Krýsa, J. et al. Photocatalytic degradation rate of oxalic acid on the semiconductive layer of n-TiO2 particles in the batch mode plate reactor Part I: Mass transfer limits. Journal of Applied Electrochemistry 28, 843–853 (1998). https://doi.org/10.1023/A:1003492510056
Issue Date:
DOI: https://doi.org/10.1023/A:1003492510056