Abstract
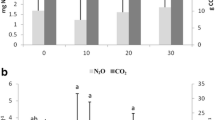
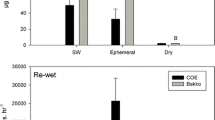
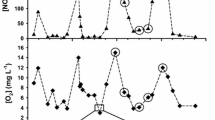
Four approaches were used to assess the effect ofinundation on methane emissions from floodplainwetlands in Victoria, Australia: (i) fieldobservations following natural rainfall events; (ii)experimental manipulation of water levels in smallfloodplain depressions; (iii) experimentalmanipulation of water levels in replicated mesocosms;and (iv) in vitro incubation of floodplainsediment under laboratory conditions. Raftery'sSwamp, a large (150 ha) wetland on the floodplain ofthe Goulburn River, became inundated in June 1993following autumn-winter rainfall. Methane emissionspeaked (1.7 ± 0.05 mmol m-2 h-1) somesix months later, and the methane content of sedimentgas bubbles reached 59% v/v, even though the positivesediment redox potentials (176 to 243 mV) indicatedthat sediments were only moderately reducing. Threesmall (< 1 ha) depressions on the floodplains of theRiver Murray and Kiewa River were inundated eithernaturally (by rain and/or overflow from nearby rivers)or artificially by flooding at specific times of year;emissions from these sites were usually negligibleafter flooding in autumn or winter. In contrast, theonset of methane emission was very rapid (within 3 to6 days) after the depressions had been flooded insummer, and the methane content of sediment gasbubbles could then reach 36% v/v. At their peak,emissions from the ephemeral wetlands were similar topeak emissions from permanent wetlands insouth-eastern Australia. Emissions from replicatedwetland mesocosms (4.5 m diameter, 0.9 m deep) werealways very small (<0.2 mmol m-2 h-1),regardless of time of flooding, water depths, orseason. In vitro incubation of wetland sedimentunder anaerobic conditions indicated a progressivedecrease in benthic methanogenesis with sedimentdesiccation and exposure to air. Ephemerallyinundated floodplain wetlands may be sites ofsignificant methane emission, especially over thesummer months. Moreover, the survival and rapidreactivation of methanogenic archaea after prolongeddrying of wetland sediments suggests thatmethanogenesis is possible even from re-wettedfloodplain environments that had earlier experiencedan extended dry phase.
Similar content being viewed by others
References
Bachoon, D. & R. D. Jones, 1992. Potential rates of methanogenesis in sawgrass marshes with peat and marl soils in the Everglades. Soil Biol. Biochem. 24: 21-27.
Bartlett, K. B., P. M. Crill, J. A. Bonassi, J. E. Richey & R. C. Harriss, 1990. Methane flux from the Amazon River floodplain: emissions during rising water. J. Geophys. Res. 95: 16,773-16,788.
Bianchi, T. S., M. E. Freer & R. G. Wetzel, 1996. Temporal and spatial variability, and the role of dissolved organic carbon (DOC) in methane fluxes from the Sabine River floodplain (southeast Texas, U.S.A.). Arch Hydrobiol. 136: 261-287.
Bohn, H. L., 1971. Redox potential. Soil Sci. 112: 39-45.
Boon, P. I., 1991. Bacterial assemblages in rivers and billabongs of southeastern Australia. Microbiol. Ecol. 22: 27-52.
Boon, P. I. & A. Mitchell, 1995. Methanogenesis in the sediments of an Australian freshwater wetland: comparison with aerobic decay, and factors controlling methanogenesis. FEMS Microbiol. Ecol. 18: 175-190.
Boon, P. I. & B. K. Sorrell, 1991. Biogeochemistry of billabong sediments. I. The effect of macrophytes. Freshwat. Biol. 26: 209- 226.
Boon, P. I. & B. K. Sorrell, 1995. Methane fluxes from an Australian floodplain wetland: the importance of emergent macrophytes. J. Nth am. Benthol. Soc. 14: 582-598.
Boon, P. I., P. Virtue & P. D. Nichols, 1996. Microbial consortia in wetland sediments: a biomarker analysis of the effects of hydrological regime, vegetation and season on benthic microbes. Mar. Freshwat. Res. 47: 27-41.
Bubier, J. L. & T. R. Moore, 1994. An ecological perspective on methane emissions from northern wetlands. Trends Ecol. Evol. 9: 460-464.
Bunn, S. E. & P. I. Boon, 1993. What sources of organic carbon drive food webs in billabongs? A study based on stable isotope analysis. Oecologia 96: 85-94.
Capone, D. G. & R. P. Kiene, 1988. Comparison ofmicrobial dynamics in marine and freshwater sediments: contrasts in anaerobic carbon metabolism. Limnol. Oceanogr. 33: 725-749.
Cappenberg, Th. E., 1974. Interrelationships between sulfate-reducing and methane-producing bacteria in bottom deposits of a fresh-waterlake. I. Field observations. Anton. van Leeuwenhoek 40: 285-295.
Chin, K.-J. & R. Conrad, 1995. Intermediary metabolism in methanogenic paddy soil and the influence of temperature. FEMS Microbiol. Ecol. 18: 85-102.
Conrad, R.H., 1995. Soil microbial processes involved in production and consumption of atmospheric trace gases. Adv. Microbiol. Ecol. 14: 207-250.
Conrad, R., H. Schutz & M. Babbel, 1987. Temperature limitation of hydrogen turnover and methanogenesis in anoxic paddy soil. FEMS Microbiol. Ecol. 45: 281-289.
Crozier, C. R., I. Devai & R. D. DeLaune, 1995. Methane and reduced sulfur gas production by fresh and dried wetland soils. Soil Sci. Soc. am. J. 59: 277-284.
Fechner-Levy, E. & H. F. Hemond, 1996. Trapped methane volume and potential effects on methane ebullition in a northern peatland. Limnol. Oceanogr. 41: 1375-1383.
Fenchel, T. & T. H. Blackburn, 1979. Bacteria and Mineral Cycling. Academic Press, London: 225 pp.
Fetzer, S., F. Bak & R. Conrad, 1993. Sensitivity of methanogenic bacteria from paddy soil to oxygen and desiccation. FEMS Microbiol. Ecol. 12: 107-115.
Finlayson, M. & M. Moser, 1991. Wetlands. International Waterfowl and Wetlands Research Bureau, Slimbridge, 224 pp.
Freeman, C., M. A. Lock & B. Reynolds, 1993. Fluxes of CO2, CH4 and N2O from a Welsh peatland following simulation of water table draw-down: potential feedback to climatic change. Biogeochemistry 19: 51-60.
Graetz, D. A., D. R. Keeney & R. B. Aspiras, 1973. Eh status of lake sediment-water systems in relation to nitrogen transformations. Limnol. Oceanogr. 18: 908-917.
Harriss, R. C., D. I. Sebacher, K. B. Bartlett, D. S. Bartlett & P. M. Crill, 1988. Sources of atmospheric methane in the south Florida environment. Global Biogeochem. Cycles 2: 231-243.
Heiler, G., T. Hein, F. Schiemer & G. Bornette, 1995. Hydrological connectivity and flood pulses as the central aspects for the integrity of a river-floodplain system. Regulated Rivers: Res. Mgmt. 11: 351-361.
Jones, J. G., 1979a. Microbial activity in lake sediments with particular reference to electrode potential gradients. J. Gen. Microbiol. 115: 19-26.
Jones, J. G., 1979b. Microbial nitrate reduction in freshwater sediments. J. Gen. Microbiol. 115: 27-35.
Jones, J. G., 1982. Activities of aerobic and anaerobic bacteria in lake sediments and their effects on the water column. In Nedwell, D. B. & C. M. Brown (eds), Sediment Microbiology. Academic Press, London: 107-145.
Jørgensen, B. B., 1977. Bacterial sulfate reduction within reduced microniches of oxidized marine sediments. Mar. Biol. 41: 7-17.
Kludze, H. K. & R. D. DeLaune, 1994. Methane emissions and growth of Spartina patensin response to soil redox intensity. Soil Sci. Soc. Am. J. 58: 1838-1845.
Lindau, C. W., W. H. Patrick Jr. & R. D. DeLaune, 1993. Factors affecting methane production in flooded rice soils. In Karl, D. M. (ed.), Agricultural Ecosystem Effects on Trace Gases and Global Climate Change. American Society for Agronomy, Madison: 157-165.
Lovley, D. R. & M. J. Klug, 1983. Sulfate reducers can outcompete methanogens at freshwater sulfate concentrations. Appl. envir. Microbiol. 45: 187-192.
Mayer, H. P. & R. Conrad, 1990. Factors influencing the population of methanogenic bacteria and the initiation of methane production upon flooding of paddy soil. FEMS Microbiol. Ecol. 73: 103-112.
Moore, T. R. & R. Knowles, 1989. The influence of water table levels on methane and carbon dioxide emissions from peatland soils. Can. J. Soil Sci. 69: 33-38.
Muller, K. L., G. G. Ganf & P. I. Boon, 1994. Methane flux from beds of Baumea arthrophylla(Nees) Boeckeler and Triglochin procerumR.Br. at Bool Lagoon, South Australia. Aust. J. Mar. Freshwat. Res. 45: 1543-1553.
Nielsen, D. L. & A. J. Chick, 1997. Flood-mediated changes in aquatic macrophyte community structure. Mar. Freshwat. Res. 48: 153-157.
Patrick, W. H. Jr. & A. Jugsujinda, 1992. Sequential reduction and oxidation of inorganic nitrogen, manganese, and iron in flooded soil. Soil Sci. Soc. Am. J. 56: 1071-1073.
Peters, V. & R. Conrad, 1995. Methanogenic and other strictly anaerobic bacteria in desert soils and other oxic soils. Appl. envir. Microbiol. 61: 1673-1676.
Pulliam, W. M., 1993. Carbon dioxide and methane exports from a southeastern floodplain swamp. Ecol. Monogr. 63: 29-53.
Reddy, K. R. & D. A. Graetz, 1988. Carbon and nitrogen dynamics in wetland soils. In Hook, D.D. et al. (eds), The Ecology and Management of Wetlands. Volume 1. Ecology of Wetlands. Croom Helm, London: 307-318.
Roden, E. E. & R. G. Wetzel, 1996. Organic carbon oxidation and suppression of methane production by microbial Fe(III) oxide reduction in vegetated and unvegetated freshwater wetland sediments. Limnol. Oceanogr. 41: 1733-1748.
Roslev, P. & G. M. King, 1996. Regulation of methane oxidation in a freshwater wetland by water table changes and anoxia. FEMS Microbiol. Ecol. 19: 105-115.
Ross, J. L., P. I. Boon, P. Ford & B. T. Hart, 1997. Detection and quantification with 16S rRNA oligonucleotide probes of plank tonic methylotrophic bacteria in a floodplain lake. Microb. Ecol. 34: 97-108.
Ryder, D. S. & P. Horwitz, 1995. Seasonal water regimes and leaf litter processing in a wetland on the Swan Coastal Plain, Western Australia. Mar. Freshwat. Res. 46: 1077-1084.
Schutz, H., W. Seiler. & R. Conrad, 1990. Influence of soil temperature on methane emissions from rice paddy fields. Biogeochemistry 11: 77-95.
Shannon, R. D. & J. R. White, 1994. A three-year study of controls on methane emissions from two Michigan peatlands. Biogeochemistry 27: 35-60.
Sorrell, B. K. & P. I. Boon, 1992. Biogeochemistry of billabong sediments. II. Seasonal variations in methane production. Freshwat. Biol. 27: 435-445.
Sorrell, B. K. & P. I. Boon, 1994. Convective gas flow in Eleocharis sphacelataR. Br.: methane transport and release from wetlands. Aquat. Bot. 47: 197-212.
Taylor, J. K., 1960. Soils. In Leper, G. W. (ed.), The Australian Environment. CSIRO and Melbourne University Press, Melbourne: 29-44.
Walker, K. F., 1985. A review of the ecological effects of river regulation in Australia. Hydrobiologia 125: 111-129.
Walker, K. F. & M. C. Thoms, 1993. Environmental effects of flow regulation on the Lower River Murray, South Australia. Regulated Rivers: Res. Mgmt. 8: 103-119.
Wang, Z., R. D. DeLaune, C. W. Lindau & W. H. Patrick, Jr., 1992. Methane production from anaerobic soil amended with rice straw and nitrogen fertilizers. Fertil. Res. 33: 115-121.
Wang, Z., C. W. Lindau, R. D. DeLaune & W. H. Patrick, Jr., 1993a. Methane emission and entrapment in flooded rice soils as affected by soil properties. Biol. Fertil. Soils 16: 163-168.
Wang, Z., R. D. DeLaune, P. H. Masscheleyn & W. H. Patrick, Jr., 1993b. Soil redox and pH effects on methane production in a flooded rice soil. Soil Sci. Soc. Am. J. 57: 382-385.
Ward, J. V. & J. A. Stanford, 1995. Ecological connectivity in alluvial river ecosystems and its disruption by flow regulation. Regulated Rivers: Res. Mgmt. 11: 105-119.
Wuebbles, D. J. & J. Edmonds, 1991. Primer on Greenhouse Gases. Lewis, Chelsea, 230 pp.
Yagi, K. & K. Minami, 1993. Spatial and temporal variations of methane flux from a rice paddy field. In Oremland, R.S. (ed.), Biogeochemistry of Global Change. Chapman and Hall, New York: 353-368.
Zeikus, J. G. & M. R. Winfrey, 1976. Temperature limitation of methanogenesis in aquatic sediments. Appl. envir. Microbiol. 31: 99-107.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Boon, P.I., Mitchell, A. & Lee, K. Effects of wetting and drying on methane emissions from ephemeral floodplain wetlands in south-eastern Australia. Hydrobiologia 357, 73–87 (1997). https://doi.org/10.1023/A:1003126601466
Issue Date:
DOI: https://doi.org/10.1023/A:1003126601466