Abstract
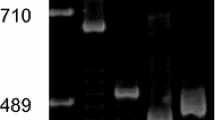
Three adjacent EcoRI fragments of the rDNA unit from Lycopersicon esculentum, eleven anonymous genomic and two anonymous cDNA clones from Brassica napus and three restriction endonucleases: BamHI, EcoRI and EcoRV were used for RFLP analysis of the genome of Hypericum perforatum L. A polymorphic band identified with EcoRI and two rDNA probes in five somaclones originated from the same genotype was detected in all progenies of two somaclones indicating the inheritance of the molecular changes. rDNA unit heterogeneity represented by two types of RFLP pattern revealed among somaclones and seed-derived control plants using EcoRV and two rDNA probes may indicate an alloploid origin of Hypericum perforatum. The occurrence of the identical RFLP patterns in some R0 somaclones and seed-derived plants and their progenies may be related to the apomictic mode of reproduction which is assumed to be prevalent in Hypericum perforatum. The differences in RFLP patterns of progenies when compared with the maternal plants (1 out of 10 progenies of one control plant and 1 out of 8 progenies of one somaclone) may indicate that some progenies have originated through sexual recombination.
Similar content being viewed by others
References
Antonius, K. & H. Nybom, 1993. DNA fingerprinting reveals significant amounts of genetic variation in a wild raspberry Rubus idaeus population. Mol Ecol 3: 177–180.
Armstrong, C.L. & R.L. Phillips, 1988. Genetic and cytogenetic variation in plants regenerated from organogenic and friable, embryogenic tissue cultures of maize. Crop Sci 28: 363–369.
Besse, P., P. Lebrun, M. Seguin & C. Lanaud, 1993. Ribosomal DNA variations in wild and cultivated rubber tree (Hevea brasiliensis). Genome 36: 1049–1057.
Breiman, A., D. Rotem-Abarbanell, A. Karp & H. Shaskin, 1987. Heritable somaclonal variation in wild barley (Hordeum spontaneum). Theor Appl Genet 74: 104–112.
Brettell, R.I.S., M.A. Pallota, J.P. Gustafson & R. Appels, 1986. Variation at the Nor loci in triticale derived from tissue culture. Theor Appl Genet 71: 637–643.
Brettell, R.I.S. & E.S. Dennis, 1991. Reactivation of a silent following tissue culture is associated with heritable alterations in its methylation pattern. Mol Gen Genet 229: 365–372.
Brown, P.T.H., E. Göbel & H. Lörz, 1991. RFLP analysis of Zea mays callus cultures and their regenerated plants. Theor Appl Genet 81: 227–232.
Chowdhury, M.K.U., V. Vasil & I.K. Vasil, 1994. Molecular analysis of plants regenerated from embryogenic cultures of wheat (Triticum aestivum L.). Theor Appl Genet 87: 821–828.
Čellárová, E., K. Kimáková & R. Brutovská, 1992. Multiple shoot formation and phenotypic changes of R0 regenerants in Hypericum perforatum L. Acta Biotechnol 12: 445–452.
Čellárová, E., Z. Daxnerová, K. Kimáková & J. Halušková, 1994. The variability of the Hypericin content in the regenerants of Hypericum perforatum. Acta Biotechnol 14: 267–274.
Čellárová, E., K. Kimáková, Z. Daxnerová & P. Mártonfi, 1995. Hypericum perforatum (St. John's wort): In vitro culture and the production of hypericin and other secondary metabolites. In: Y.P.S. Bajaj (Ed.), Biotechnology in Agriculture and Forestry 33, Medicinal and Aromatic Plants VIII, pp, 261–275. Springer-Verlag, Berlin.
Delseny, M., J.M. McGrath, P. This, A.M. Cherre & C.F. Quiros, 1990. Ribosomal RNA genes in diploid and amphidiploid Brassica and related species: organization, polymorphisms and evolution. Genome 33: 733–744.
Deumling, B. & L. Clermont, 1989. Changes in DNA content and chromosomal size during cell culture and plant regeneration of Scilla siberica: selective chromatin diminuation in response to environmental conditions. Chromosoma 97: 439–448.
Diwu, Z. & J.W. Lown, 1994. Phototherapeutic potential of alternative photosenzitizers to porphyrins. Pharmac Ther 63: 1–35.
Feinberg, A.P. & B. Vogelstein, 1983. A technique for radiolabelling DNA restriction endonuclease fragments to high specific activity. Anal Biochem 132: 6–13.
Gerstel, D.V. & W. Mishanec, 1950. On the inheritance of apomixis in Parthenium argentatum. Bot Gaz 112: 96–106.
Holmes, D.S. & M. Quigley, 1981. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem 114: 193–197.
Kaeppler, S.M., 1992. Molecular and genetic studies of tissue culture-induced variation in maize. St. Paul: Univ Minnesota, Thesis.
Kiss, T., M. Kis, S. Abel & F. Solomosy, 1988. Nucleotide sequence of the 17S–25S spacer region from tomato rDNA. Nucl Acid Res 16: 7179.
Kiss, T., M. Kis & F. Solomosy, 1989. Nucleotide sequence of a 25S-rDNA gene from tomato. Nucl Acid Res 17: 796.
Larkin, P.J. & W.R. Scowcroft, 1981. Somaclonal variation — a novel source of variability from cell cultures for plant improvement. Theor Appl Genet 60: 197–214.
Lee, M. & R.L. Phillips, 1988. Genetic variants in progeny of regenerated maize plants. Genome 29: 834–838.
Levall, M.W., K. Bengtsson, N.-O. Nilsson, A. Hjerdin & C. Halldén, 1994. Molecular characterization of UV-treated sugar beet somaclones using RFLP markers. Physiol Plant 90: 216–220.
Linsmaier, E.M. & F. Skoog, 1965. Organic factor requirement of tobacco tissue cultures. Physiol Plant 18: 100–127.
Maniatis, T., E.F. Fritsch & J. Sambrook, 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Müller, E., P.T.H. Brown, S. Hartke & H. Lörz, 1990. DNA variation in tissue culture-derived rice plants. Theor Appl Genet 80: 673–679.
Noack, K.L., 1939. Über Hypericum — Kreuzungen VI. Fortpflanzungsverhältnisse und Bastarde von Hypericum perforatum L. Z Indukt Abstamm Vererbungslehre 76: 569–601.
Nybom, H., 1993. Application of DNA fingerprinting in plant population studies. In: S.D.J. Pena, R. Chakraborty, J.T. Epplen & A.J. Jeffreys (Eds.), DNA Fingerprinting: State of the Science, pp. 293–309. Birkhäser Verlag, Basel.
Peschke, V.M., R.L. Phillips & B.G. Gengenbach, 1991. Genetic and molecular analysis of tissue culture-derived Ac elements. Theor Appl Genet 82: 121–129.
Robson, N.K.B., 1981. Studies in the genus Hypericum L. (Guttiferae). 2. Characters of the genus. Bull Br Mus Nat Hist Bot 8: 55–226.
Saghai-Maroof, M.A., K.M. Soliman, R.A. Jorgensen & R.W. Allard, 1984. Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc Natl Acad Sci USA 81: 8014–8018.
Zheng, K.L., S. Castiglione, M.G. Biasini, A. Biroli, C. Morandi & F. Sala, 1987. Nuclear DNA amplification in cultured cells of Oryza sativa L. Theor Appl Genet 74: 65–70.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Halušková, J., Čellárová, E. RFLP analysis of Hypericum perforatum L. somaclones and their progenies. Euphytica 95, 229–235 (1997). https://doi.org/10.1023/A:1002946618273
Issue Date:
DOI: https://doi.org/10.1023/A:1002946618273