Abstract
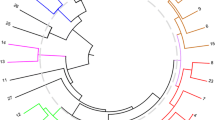
A collection of 64 fig (Ficus carica L.) accessions was characterized through the use of RAPD markers, and results were evaluated in conjunction with morphological and agronomical characters, in order to determine the genetic relatedness of genotypes with diverse geographic origin. The results indicate that fig cultivars have a rather narrow genetic base. Nevertheless, RAPD markers could detect enough polymorphism to differentiate even closely related genotypes (i.e., clones of the same cultivar) and a unique fingerprint for each of the genotypes studied was obtained. No wasteful duplications were found in the collection. Cluster analysis allowed the identification of groups in accordance with geographic origin, phenotypic data and pedigree. Taking into account the limited information concerning fig cultivar development, the results of this study, which provide information on the genetic relationships of genetically distinct material, dramatically increase the fundamental and practical value of the collection and represent an invaluable tool for fig germplasm management.
Similar content being viewed by others
References
Apostol, B.L., W.C. Black, B.R. Miller & P. Reiter, 1996. Population genetics with RAPD-PCR markers: the breeding structure of Aedes aegypti in Puerto Rico. Heredity 76: 325–334.
Armstrong, J., A. Gibbs, R. Peakall & G. Weiler, 1996. RAPDistance programs: version 1.04 for the analysis of patterns of RAPD fragments. Australian National University, Canberra.
Beck, N.G. & E.M. Lord, 1988a. Breeding system in Ficus carica, the common fig. I. Floral diversity. Am. J. Bot. 75: 1906–1912.
Beck, N.G. & E.M. Lord, 1988b. Breeding system in Ficus carica, the common fig. II. Pollination events. Am. J. Bot. 75: 1913–1922.
Cabrita, L.F., U. Aksoy, S. Hepaksoy & J.M. Leitao, 2001. Suitability of isozyme, RAPD and AFLP markers to assess genetic differences and relatedness among fig (Ficus carica L.) clones. Scientia Horticulturae 87: 261–273.
Cattan-Toupance, I., Y. Michalak is & C. Neema, 1998. Genetic structure of wild bean populations in their South-Andean centre of origin. Theor. Appl. Genet. 96: 844–851.
Condit, I.J., 1932. The stucture and development of flowers in Ficus carica. Hilgardia 6: 443–481.
Condit, I.J., 1941. Fig characteristics useful in the identification of varieties. Hilgardia 14: 1–69.
Condit, I.J., 1955. Fig varieties: a monograph. Hilgardia 23: 322–538.
Condit, I.J., 1956. Promising new seedling fig. Calif. Agr. 10: 4–14.
Crouch, J.H., H.K. Crouch, H. Constandt, A.V. Gysel, P. Breyne, M.V. Montagu, R.L. Jarret & R. Ortiz, 1999. Comparison of PCR-based molecular marker analyses of Musa breeding populations. Molec. Breed. 5: 233–244.
Dunemann, G., R. Kahnau & H. Schmidt, 1994. Genetic relationships in Malus evaluated by RAPD fingerprinting of cultivars and wild species. Plant Breed. 113: 150–159.
Felsenstein, J., 1993. PHYLIP: Phylogeny Inference Package, version 3.75c. Department of Genetics, University of Washington, Seattle, USA.
Ferguson, L., T.J. Michailides & H. Shorey, 1990. The California fig industry. Hort. Rev. 12: 409–490.
Galderisi, U., M. Cipollaro, G.D. Bernardo, L. De Masi, G. Galano & A. Cascino, 1999. Identification of the edible fig ‘Bianco del Cilento’ by random amplified polymorphic DNA analysis. HortSciense 34(7): 1263–1265.
Gardiner, S.E., H.C.M. Basset, D. Madie & D.A.M. Noiton, 1996. Isozyme, radomly amplified polymorphic DNA (RAPD) and restriction fragment lenth polymorphism (RFLP) markers use dto deduce a putative parent for the ‘Braeburn’ apple. J. Amer. Soc. Hort. Sci. 121: 996–1001.
Gibert, J.E., R.V. Lewis, M.J. Wilkinson & P.D.S. Caligari, 1999. Developing an appropriate strategy to assess genetic variability in germplasm collections. Theor. Appl. Genet. 98: 1125–1131.
Gutman, F., A. Nerd, Y. Mizrahi, D. Bar-Zvi & D. Raveh, 1999. Application of random amplified polymorphic DNA markers for identification of Marula genotypes. HortSciense 34(7): 1256–1258.
Hallden, C., N.O. Nilsson, I.M. Rading & T. Sall, 1994. Evaluation of RFLP and RAPD markers in a comparison of Brassica napus breeding lines. Theor. Appl. Genet. 88: 123–128.
Jaccard, P., 1908. Nouvelles recherches sur la distribution florale. Bull. Soc. Vaud. Sci. Nat. 44: 223–270.
Kazan, K., J.M. Manners & D.F. Cameron, 1993. Genetic variation in agronomically important species of Stylosanthes determined using random ampified polymorphic DNA markers. Theor. Appl. Genet. 85: 882–888.
Khadari, B., P. Lashermes & F. Kjellberg, 1995. RAPD fingerprints for identification and genetic characterization of fig (Ficus carica L.) genotypes. J. Genet. Breed. 49: 77–86.
Kjellberg, F., P.H. Gouyon, M. Ibrahim, M. Raymond & G. Valdeyron, 1987. The stability of the symbiosis between dioecious figs and their pollinators: a study of Ficus carica L. and Blastophaga psenes L. Evolution 41: 693–704.
Koller, B., A. Lehmann, J.M. McDermott & C. Gesser, 1993. Identification of apple cultivars using RAPD markers. Theor. Appl. Genet. 85: 901–904.
Kresovich, S., J.G.K. Williams, J.R. McFerson, E.J. Routman & B.A. Schaal, 1992. Characterization of genetic identities and relationships of Brassica oleracea L.via a random amplified polymorphic DNA assay. Theor. Appl. Genet. 85: 190–196.
Lakshmi, M., S. Rajalakshmi, M. Parami, C.S. Anuratha & A. Parida, 1997. Molecular phylogeny of mangroves. I. Use of molecular markers in assessing the intraspecific genetic variability in the mangrove species Acanthus ilicifolius Linn. (Acanthaceae). Theor. Appl. Genet. 94: 1121–1127.
Lefebvre, V., B. Goffinet, J.C. Chauvet, B. Caromel, P. Signoret, R. Brand & A. Palloix, 2001. Evaluation of genetic distances between pepper inbred lines for cultivar protection purposes: comparison of AFLP, RAPD and phenotypic data. Theor. Appl. Genet. 102: 741–750.
Lionakis, S.M., 1994. Present status and future prospects of the cultivation in Greece of the plants: fig, persimmon, pomegranate and barbary fig, pp. 14–21 in Proc. First Meeting of the CIHEAM Cooperative Research Network on Underutilized Fruit Trees, Zaragosa.
McRoberts, N., R.P. Finch, W. Sinclair, A. Meikle, G. Marshall, G. Squire & J. McNicol, 1999. Accessing the ecological significance of molecular diversity data in natural plant populations. J. Exp. Bot. 50: 1635–1645.
Metais, I., C. Aubry, B. Hamon, R. Jalouzot & D. Peltier, 2000. Description and analysis of genetic diversity between commercial bean lines (Phaseolus vulgaris L.). Theor. Appl. Genet. 101: 1207–1214.
Nebauer, S.G., L.D. Castillo-Agudo & J. Segura, 1999. RAPD variation within and among natural populations of outcrossing willow-laeved foxglove (Digitalis obscura L.). Theor. Appl. Genet. 98: 985–994.
Nelson, C.D., W.L. Nance & R.L. Doudrick, 1993. A partial genetic linkage map of slash pine (Pinus elliottii Engelm. var.elliottii based on random amplified polymorphic DNAs. Theor. Appl. Genet. 87: 145–151.
Nicese, F.P., J.I. Hormaza & G.H. McGranahan, 1998. Molecular characterization and genetic relatedness among walnut (Juglans regia L.) genotypes based on RAPD markers. Euphytica 101: 199–206.
Obenauf, G., M. Gerdts, G. Leavitt & J. Crane, 1978. Commercial dried fig production in California, pp. 1–31 in California, Agricultural Scienses. University of California, Leaflet 21051.
Owuor, E.D., T. Fahima, A. Beharav, A. Korol & E. Nevo, 1999. RAPD divergence caused by microsite edaphic selection in wild barley. Genetica 105: 177–192.
Palacios, C. & F. Gonzalez-Candelas, 1997. Analyis of population genetic structure and variability using RAPD markers in the endemic and endangered Limonium dufourii (Plumbaginaceae). Mol. Ecol. 6: 1107–1121.
Papa, R., G. Attene, G. Barcaccia, A. Ohgata & T. Konishi, 1998. Genetic diversity in landrace polulations of Hordeum vulgare L. from Sardinia, Italy, as revealed by RAPDs, isozymes and morphophenological traits. Plant Breed. 117: 523–530.
Rodriguez, J.M., T. Berke, L. Engle & J. Nienhuis, 1999. Variation among and within Capsicum species revealed by RAPD markers. Theor. Appl. Genet. 99: 147–156.
Rogers, S.O. & A.J. Bendich, 1988. Extraction of DNA from plant tissues, pp. 1–11 in Plant Molecular Biology Manual, edited by S.B. Gevin, R.A. Schilperoort & D.P.S. Verma. Kluwer Academic Publishers, Dordrecht,.
Sadhu, M.K., 1990. Fig, pp. 650–663 in Fruits: Tropical and Subtropical, edited by T.K. Kose & S.K. Mitra. Naya Prokash, Calcutta.
Song, B.K., M.M. Clyde, R. Wickneswari & M.N. Normah, 2000. Genetic Relatedness among Lansium domesticum accessions using RAPD markers. Ann. Bot. 86: 299–307.
Storey, W.B., 1975. Figs, pp. 568–589 in Advances in Fruit Breeding, edited by J. Janick & J.N. Moore. Purdue Univ. Press, W. Lafayette, Indiana.
Tous, J. & L. Ferguson, 1996. Mediterranean fruits, pp. 416–430 in Progress in New Crops, edited by J. Janick. ASHS Press, Arlington, V.A.
Valdeyron, G. & D.G. Lloyd, 1979. Sex differences and flowering phenology in the common fig, Ficus carica L. Evolution 33: 673–675.
Wang, M. & I.L. Goldman, 1999. Genetic distance and diversity in table beet and sugar beet accessions measured by randomly amplified polymorphic DNA. J. Amer. Soc. Hort. Sci. 124: 630–635.
Welsh, J. & M. McClelland, 1990. Fingerprinting genomes using PCR with arbitrary primers. Nucl. Acids Res. 18: 7213–7218.
Wiesman, Z., N. Avidan, S. Lavee & B. Quebedeaux, 1998. Molecular characterization of common olive varieties in Israel and the West Bank using randomly amplified polymorphic DNA (RAPD) markers. J. Amer. Soc. Hort. Sci. 123: 837–841.
Williams, J.G.K., A.R. Kubelik, K.I. Livak, J.A. Rafalski & S.V. Tingey, 1990. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucl. Acids Res. 18: 6531–6535.
Yang, X. & C. Quiros, 1993. Identification and classification of celery cultivars with RAPD markers. Theor. Appl. Genet. 86: 205–212.
Zohary, D. & M. Hopf, 1988. Domestication of Plants in the Old World: the Origin and Cultivated Plants in West Asia, Europe and the Nile Valley. Oxford University Press, New York.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Papadopoulou, K., Ehaliotis, C., Tourna, M. et al. Genetic Relatedness Among Dioecious Ficus carica L. cultivars by Random Amplified Polymorphic DNA Analysis, and Evaluation of Agronomic and Morphological Characters. Genetica 114, 183–194 (2002). https://doi.org/10.1023/A:1015126319534
Issue Date:
DOI: https://doi.org/10.1023/A:1015126319534