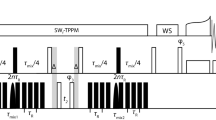
Abstract
Recently, several TROSY-based experiments have been designed for backbone chemical shift assignment and measurement of the NOEs of 2H, 13C and 15N labeled proteins. Here, we present TROSY-enhanced NOESY experiments, namely the 2D S3E-NOESY-S3E, 3D TROSY-NOESY-S3E and S3E-NOESY-TROSY experiments. These experiments use the spin-state selective excitation method (S3E), and have the TROSY effect in all the indirectly and directly detected dimensions, and so provide optimal resolution for amide protons. The first two experiments provide an additional useful feature in that the diagonal peaks of the amide proton region are cancelled or greatly reduced, allowing clear identification of NOE cross peaks that are close to diagonal peaks.
Similar content being viewed by others
References
Brutscher, B., Boisbouvier, J., Pardi, A., Marion, D. and Simorre, J.P. (1998) J. Am. Chem. Soc., 120, 11845–11851.
Delaglio, F., Grzesiek, S., Vuister, G.W., Zhu, G., Pfeifer, J. and Bax, A. (1995) J. Biomol. NMR, 6, 277–293.
Ishima, R., Wingfield, P.T., Stahl, S.J., Kaufman, J.D. and Torchia, D.A. (1998) J. Am. Chem. Soc., 120, 10534–10542.
Meissner, A., Duus, J.Ø. and Sørensen, O.W. (1997) J. Biomol. NMR, 10, 89–94.
Nietlispach, D., Clowes, R.T., Broadhurst, R.W., Ito, Y., Keeler, J., Kelly, M., Ashurst, J., Oschkinat, H., Domaille, P.J. and Laue, E.D. (1996) J. Am. Chem. Soc., 118, 407–415.
Pervushin, K., Riek, R., Wider, G. and Wüthrich, K. (1997) Proc. Natl. Acad. Sci. USA, 94, 12366–12371.
Pervushin, K., Riek, R., Wider, G. and Wüthrich, K. (1998) J. Am. Chem. Soc., 120, 6394–6400.
Reisman, J., Jariel-Encontre, I., Hsu, V.L., Parello, J., Geiduschek, E.P. and Kearns, D.R. (1991) J. Am. Chem. Soc., 113, 2787–2789.
Salzmann, M., Pervushin, K., Wider, G., Senn, H. and Wüthrich, K. (1998) Proc. Natl. Acad. Sci. USA, 95, 13585–13590.
Sørensen, M.D., Meissner, A. and Sørensen, O.W. (1997) J. Biomol. NMR, 10, 181–186.
States, D.I., Haberkorn, R.A. and Ruben, D.J. (1982) J. Magn. Reson., 93, 151.
Weigelt, J. (1998) J. Am. Chem. Soc., 120, 10778–10779.
Yang, D. and Kay, L.E. (1999) J. Biomol. NMR, 13, 3–10.
Zhu, G., Kong, X.M., Yan, X.Z. and Sze, K.H. (1998) Angew. Chem. Int. Ed. Engl., 37, 2859–2861.
Zhu, G., Kong, X.M. and Sze, K.H. (1999) J. Biomol. NMR, 13, 77–81.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhu, G., Xia, Y., Sze, K.H. et al. 2D and 3D TROSY-enhanced NOESY of 15N labeled proteins. J Biomol NMR 14, 377–381 (1999). https://doi.org/10.1023/A:1008386525465
Issue Date:
DOI: https://doi.org/10.1023/A:1008386525465