Abstract
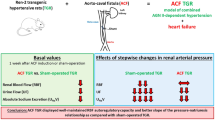
Altered regulation of cAMP may contribute to enhanced renal reactivity to angiotensin II (Ang II) in spontaneously hypertensive rats (SHR). Such a phenomenon may occur in renal preglomerular arterioles and may involve changes in expression of GTP-binding regulatory proteins. We have examined the effects of Ang II on steady state levels of Gαi-1,2, Gαi-3 Gαs and Gαq in preglomerular arterioles from young marginally hypertensive SHR and on mean arterial pressure (MAP), renal vascular resistance (RVR) and renal cAMP excretion (UcAMP.V). Young (5-6 week old) SHR and Wistar Kyoto (WKY) rats received Ang II (35 ng/kg/min, s.c.) or vehicle for 7 days via osmotic minipumps. Urine was collected over the last 24 h. On day seven, MAP and renal blood flow were measured in anesthetized rats and RVR was determined. Preglomerular arterioles were isolated by perfusing the kidneys with iron oxide and using a series of mechanical steps coupled with the use of a magnet to retain iron-laden vessels. Membranes were prepared and the expressions of Gαi-1,2, Gαi-3, Gαs and Gαq were evaluated by Western immunoblotting. Baseline MAP (124 ± 6 mmHg) was only marginally (p > 0.05) higher in SHR when compared with WKY rats (110 ± 4 mmHg). RBF (3.04 ± 0.16 mL/min) was significantly lower and RVR (41.10 ± 1.37 mmHg.min/mL) was significantly higher in SHR when compared to age-matched WKY rats (4.36 ± 0.30 mL/min and 25.79 ± 1.58 mmHg.min/mL, respectively). Ang II significantly increased MAP in SHR (17 mmHg) but not in WKY rats. These increases in MAP were accompanied by significant increases in RVR in SHR (48% over control) but not in WKY rats. Compared to WKY rats, preglomerular arterioles from SHR exhibited significantly higher basal expression of Gαi-1,2 (11- fold), Gαi-3 (13-fold) and Gαs (3-fold). Chronic infusion of Ang II, however, downregulated the expression of Gαs (by 53%; p < 0.05), Gαi-1,2 ( by 72%; p < 0.05) and Gαi-3 (by 35%; p > 0.05) in SHR preglomerular arterioles but significantly upregulated the expression of these proteins in WKY by 3-, 8- and 15-fold, respectively. Basal levels of Gαq were not different in preglomerular arterioles from the two strains but were downregulated by Ang II in both WKY (74% of basal) and SHR (52% of control). Baseline UcAMP.V was significantly lower in SHR (31.22 ± 6.51 nmol/24 h) compared with WKY rats (65.33 ± 3.60 nmol/24 h). Chronic Ang II infusion significantly increased UcAMP.V in SHR as well as WKY rats. These data clearly demonstrate that expressions of Gi isoforms as well as Gs in renal microvessels are elevated during early stages of hypertension and suggest that the elevated levels of Gi proteins may be directly associated with a blunted adenylyl cyclase-cAMP cascade in the renal microvasculature. Furthermore, Ang II appears to directly downregulate the expression of Gs in young SHR but not in young WKY renal microvessels. Such diversity in its effect on G-protein expression may be important for enhanced renal sensitivity to Ang II in SHR.
Similar content being viewed by others
References
Li P, Jackson EK: Enhanced slow-pressor response to angiotensin II in spontaneously hypertensive rats. J Pharmacol Exp Ther 251: 909–921, 1989
Chatziantoniou C, Arendshorst WJ: Angiotensin and thromboxane in genetically hypertensive rats: Renal blood flow and receptor studies. Am J Physiol 261: F238–F247, 1991
Kost CK Jr, Jackson EK: Enhanced renal angiotensin II subtype 1 receptor responses in the spontaneously hypertensive rat. Hypertension 21: 420–431, 1993
Chatziantoniou C, Daniels FH, Arendshorst WJ: Exaggerated renal vascular reactivity to angiotensin and thromboxane in young genetically hypertensive rats. Am J Physiol 259: F372–F382, 1990
Vyas SJ, Jackson EK: Angiotensin II: Enhanced renal responsiveness in young genetically hypertensive rats. J Pharmacol Exp Ther 273:768–777, 1995
Ljungman S, Aurell M, Hartford M, Wikstrand J, Berglund G: Effects of subpressor doses of angiotensin II on renal hemodynamics in relation to blood pressure. Hypertension 5: 368–374, 1983
Widgren BR, Herlitz H, Aurell M, Berglund G, Wikstrand J, Andersson OK: Increased systemic and renal vascular sensitivity to angiotensin II in normotensive men with positive family histories of hypertension. Am J Hypertens 5: 167–174, 1992
Vyas SJ, Mi Z, Jackson EK: The inhibitory effect of angiotensin II on stimulus-induced release of cAMP is augmented in the genetically hypertensive rat kidney. J Pharmacol Exp Ther 279: 114–119, 1996
Jackson EK: Pertussis toxin normalizes enhanced renovascular responses to angiotensin II in spontaneously hypertensive rats. Life Sci 54: 445–450, 1994
Vyas SJ, Mokkapatti R, Dubey RK, Chinoy MR, Jackson EK: Guanine nucleotide-binding inhibitory protein-mediated inhibition of adenylyl cyclase is enhanced in spontaneously hypertensive rat preglomerular arteriolar smooth muscle cells. J Pharmacol Exp Ther 285: 828–834, 1998
Thibault C, Anand-Srivastava MB: Altered expression of G-protein mRNA in spontaneously hypertensive rats. FEBS Lett 313: 160–164, 1992
Anand-Srivastava MB: G-proteins and adenylyl cyclase signalling in hypertension. Mol Cell Biochem 157: 163–170, 1996
Anand-Srivastava MB: Enhanced expression of inhibitory guanine nucleotide regulatory protein in spontaneously hypertensive rats. Relationship to adenylate cyclase inhibition. Biochem J 288: 79–85, 1992
Bohm M, Gierschik P, Knorr A, Larisch K, Weismann K, Erdmann E: Role of altered G-protein expression in the regulation of myocardial adenylyl cyclase activity and force of contraction in spontaneous hypertensive cardiomyopathy in rats. J Hypertens 10: 1115–1128, 1992
Lustig KD, Conklin BR, Herzmark P, Taussig R, Bourne HR: Type II adenylyl cyclase integrates coincident signals from Gs, Gi, and Gq. J Biol Chem 268: 13900–13905, 1993
Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976
Blewett CJ, Zgleszewski SE, Chinoy MR, Krummel TM, Cilley RE: Bronchial ligation enhances murine fetal lung development in wholeorgan culture. J Pediatr Surg 31: 869–877, 1996
Laemmli UK: Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 227: 680–685, 1970
Beierwaltes WH, Arendshorst WA, Klemmer PJ: Electrolyte and water balance in young spontaneously hypertensive rats. Hypertension 4: 908–915, 1982
Dilley JR, Arendshorst WJ: Enhanced tubuloglomerular feedback activity in rats developing spontaneous hypertension. Am J Physiol 247: F672–F679, 1984
Dilley JR, Stier CT Jr, Arendshorst WJ: Abnormalities in glomerular function in rats developing spontaneous hypertension. Am J Physiol 246: F12–F20, 1984
Harrap SB, Doyle AE: Renal hemodynamics and total body sodium in immature spontaneously hypertensive and Wistar-Kyoto rats. J Hypertens 4: S249–S252, 1986
Weiss RH: G protein-coupled receptor signalling in the kidney. Cell Signal 10: 313–320, 1998
Marcil J, Champlain J, Anand-Srivastava MB: Overexpression of Giproteins precedes the development of DOCA-salt-induced hypertension: Relationship with adenylyl cyclase. Cardiovasc Res 39: 492–505, 1998
Vyas SJ, Blaschak CM, Chinoy MR, Jackson EK: Angiotensin II-induced alterations in GTP-binding regulatory proteins in renal preglomerular arterioles from genetically hypertensive rats. Am J Hypertens 12: 102A, 1999
Mokkapatti R, Vyas SJ, Jackson EK: G protein mRNA expression in renal microvessels from spontaneously hypertensive and Wistar-Kyoto rats. Am J Physiol 273: F877–F882, 1997
Gilman AG: G proteins and dual control of adenylylate cyclase. Cell 36: 577–579, 1984
Sunahara RK, Dessauer CW, Gilman AG: Complexity and diversity of mammalian adenylyl cyclases. Annu Rev Pharmacol Toxicol 36: 461–480, 1996
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vyas, S.J., Blaschak, C.M., Chinoy, M.R. et al. Angiotensin II-induced changes in G-protein expression and resistance of renal microvessels in young genetically hypertensive rats. Mol Cell Biochem 212, 121–129 (2000). https://doi.org/10.1023/A:1007125525858
Issue Date:
DOI: https://doi.org/10.1023/A:1007125525858