Abstract
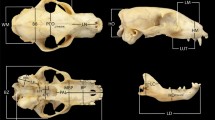
We report on intraspecific and interspecific morphological variation in the cranium, mandible and teeth along the ontogenetic trajectories of the two species of the largest living rodent, the capybara. A three dimensional geometric morphometrics approach was used to compare 171 Hydrochoerus hydrochaeris and 44 Hydrochoerus isthmius specimens ranging from newborn to adult. The specimens were assigned to seven different age classes according to cranial suture closure. The species can be differentiated in the morphospace occupation. They differ in the angle between the braincase and rostrum—H. hydrochaeris displays a straight transition whereas the snout of H. isthmius is inclined ventrally. The males in both species are bigger than the females, but no shape differences were detected. The youngest two age classes (up to 0.5 months and 0.5–10 months; before reaching sexual maturity) can be morphologically differentiated from the older age classes. Shape changes during growth are similar in both species: with increasing age, the round neurocranium flattens and the proportionally short snout elongates. Moreover, both species follow similar ontogenetic trajectories. H. hydrochaeris and H. isthmius can be differentiated by size and shape; the shape differences may indicate differences in diet and habitat. This study illustrates the relevance of an ontogenetic perspective to characterize species and examine the bases of disparity in adults. Furthermore, variation recorded in dental features serves to evaluate taxonomic and evolutionary aspects in fossil capybaras.
Similar content being viewed by others
References
Adams, D.C., Otárola-Castillo, E., 2013. Geomorph: an R package for the collection and analysis of geometric morphometric shape data. Methods Ecol. Evol. 4, 393–399, https://doi.org/10.1111/2041-210X.12035.
Álvarez, A., Pérez, S.I., Verzi, D.H., 2015. The role of evolutionary integration in the morphological evolution of the skull of caviomorph rodents (Rodentia: Hystricomorpha). Evol. Biol. 42(3), 312–327, https://doi.org/10.1007/s11692-015-9326-7.
Barreto, G.R., Quintana, R.D., 2013. Foraging strategies and feeding habits of capybaras. In: Moreira, J.R., Ferraz, K.M.P.M.B., Herrera, E.A., Macdonald, D.W. (Eds.), Capybara: Biology, Use and Conservation of an Exceptional Neotropical Species. Springer Science & Business Media, pp. 83–96.
Bookstein, F.L., 1991. Morphometric Tools for Landmark Data. Cambridge University Press, Cambridge.
Cardini, A., O’Higgins, P., 2004. Patterns of morphological evolution in Marmota (Rodentia, Sciuridae): geometric morphometrics of the cranium in the context of marmot phylogeny, ecology and conservation. Biol. J. Linn. Soc. 82, 385–407, https://doi.org/10.1111/j.10958312.2004.00367.x.
Cardini, A., Polly, P.D., 2013. Larger mammals have longer faces because of size-related constraints on skull form. Nat. Commun. 4, https://doi.org/10.1038/ncomms3458.
Christiansen, P., 2012. The making of a monster: postnatal ontogenetic changes in craniomandibular shape in the great sabercat Smilodon. PLoS One 7 (1), e29699, https://doi.org/10.1371/journal.pone.0029699.
Carrillo, J.D., Sánchez-Villagra, M.R., 2015. Giant rodents from the Neotropics: diversity and dental variation of late Miocene neoepiblemid remains from Urumaco, Venezuela. Paläontologische Zeitschrift 89, 1057–1071, https://doi.org/10.1007/s12542-015-0267-3.
Clauss, M., Dittmann, M.T., Müller, D.W.H., Zerbe, P., Codron, D., 2014. Low scaling of a life history variable: analysing eutherian gestation periods with and without phylogeny-informed statistics. Mamm. Biol. 79 (1), 9–16.
Deschamps, CM., Olivares, I., Vieytes, E.C., Vucetich, M.G., 2007. Ontogeny and diversity of the oldest capybaras (Rodentia: Hydrochoeridae; late Miocene of Argentina). J. Vertebr. Paleontol. 27, 683–692, https://doi.org/10.1671/02724634(2007) 27[683:OADOTO]2.0.CO;2.
Deschamps, CM., Vucetich, M.G., Montalvo, C.I., Zárate, M.A., 2013. Capybaras (Rodentia, Hydrochoeridae, Hydrochoerinae) and their bearing in the calibration of the late Miocene-Pliocene sequences of South America. J. S. Am. Earth Sci. 48, 145–158, https://doi.org/10.1016/jjsames.2013.09.007.
Drake, A.G., Klingenberg, C.P., 2008. The pace of morphological change: historical transformation of skull shape in St Bernard dogs. Proc. R. Soc. Lond. B: Biol. Sci. 275, 71–76, https://doi.org/10.1098/rspb.2007.1169.
Farmer, M.A., German, R.Z., 2004. Sexual dimorphism in the craniofacial growth of the guinea pig (Cavia porcellus). J. Morphol. 259, 172–181, https://doi.org/10.1002/jmor.10180.
Flores, D.A., Abdala, F., Giannini, N., 2010. Cranial ontogeny of Caluromys philander (Didelphidae: Caluromyinae): a qualitative and quantitative approach. J. Mamm. 91 (3), 539–550, https://doi.org/10.1644/09-mamm-a-291.1.
Flores, D.A., Abdala, F., Martin, G.M., Giannini, N.P., Martinez, J.M., 2015. Post-weaning growth in shrew opossums (Caenolestidae): a comparison with bandicoots (Peramelidae) and carnivorous marsupials. J. Mamm. Evol. 22, 285–303, https://doi.org/10.1007/s10914014-9279-0.
Fuchs, M., Geiger, M., Stange, M., Sánchez-Villagra, M.R., 2015. Growth trajectories in the cave bear and its extant relatives: an examination of ontogenetic patterns in phylogeny. BMC Evol. Biol. 15, 239, 10.1186/s12862–015–0521-z.
Goodall, C., 1991. Procrustes methods in the statistical analysis of shape. J. R. Stat. Soc. Ser. B (Methodol.), 285–339.
Hautier, L., Lebrun, R., Cox, P.G., 2012. Patterns of covariation in the masticatory apparatus of hystricognathous rodents: implications for evolution and diversification.J. Morphol. 273, 1319–1337, https://doi.org/10.1002/jmor.20061.
Herrel, A., Fabre, A.-C, Hugot, J.P., Keovichit, K., Adriaens, D., Brabant, L., Van Hoorebeke, L., Cornette, R., 2012. Ontogeny of the cranial system in Laonastes aenigmamus. J. Anat. 221, 128–137, https://doi.org/10.1111/j.1469-7580.2012.01519.x.
Herring, S.W., 1993. Formation of the vertebrate face: epigenetic and functional influences. Am. Zool. 33 (4), 472–483, https://doi.org/10.1093/icb/33.4.472.
Hughes, P.C.R., Tanner, J.M., Williams, J.P.G., 1978. A longitudinal radiographic study of the growth of the rat skull. J. Anat. 127, 83–91.
Hulbert, R.C., 2001. Mammalia 4-rodents and Lagomorpha. In: Hulbert, R.C. (Ed.), The Fossil Vertebrates of Florida. University Press of Florida, p. 226.
Kerber, L., Ribeiro, A.M., 2011. Capybaras (Rodentia: Hystricognathi: Hydrochoeridae) from the late Pleistocene of southern Brazil. Neues Jahrbuch fürGeologie und Paläontologie Abhandlungen 261, 1–18 https://doi.org/10.1127/0077-7749/2011/0142.
Klingenberg, C.P., 2011. MorphoJ: an integrated software package for geometric morphometrics. Mol. Ecol. Res. 11, 353–357, https://doi.org/10.1111/j.1755-0998.2010.02924.x.
Klingenberg, C.P., Marugán-Lobón, J., 2013. Evolutionary covariation in geometric morphometric data: analyzing integration, modularity, and allometry in a phylogenetic context. Syst. Biol. 62, 591–610, https://doi.org/10.1093/sysbio/syt025.
Klingenberg, C.P., McIntyre, G.S., 1998. Geometric morphometrics of developmental instability: analyzing patterns of fluctuating asymmetry with Procrustes methods. Evolution, 1363–1375, https://doi.org/10.2307/2411306.
Kolb, C., Scheyer, T.M., Lister, A.M., Azorit, C., de Vos, J., Schlingemann, M.A., Rössner, G.E., Monaghan, N.T., Sánchez-Villagra, M.R., 2015. Growth in fossil and extant deer and implications for body size and life history evolution. BMC Evol. Biol. 15, 19, https://doi.org/10.1186/s12862-015-0295-3.
Kruska, D., 1970. Veränderungendes Zentralnervensystems vonWild-und Hausschwein. Zeitschrift Anatomie Entwicklungsgeschichte 131, 291–324.
Kruska, D., 1996. The effect of domestication on brain size and composition in the mink (Mustela vison). J. Zool. 239, 645–661, https://doi.org/10.1111/j.14697998.1996.tb05468.x.
La Croix, S., Holekamp, K.E., Shivik, J.A., Lundrigan, B.L., Zelditch, M.L., 2011. Ontogenetic relationships between cranium and mandible in coyotes and hyenas. J. Morphol. 272, 662–674, https://doi.org/10.1002/jmor.10934.
Lawing, A.M., Polly, P.D., 2010. Geometric morphometrics: recent applications to the study of evolution and development. J. Zool. 280, 1–7.
Mitteroecker, P., Gunz, P., Bernhard, M., Schaefer, K., Bookstein, F.L., 2004. Comparison of cranial ontogenetic trajectories among great apes and humans. J. Hum. Evol. 46, 679–698, https://doi.org/10.1016/jjhevol.2004.03.006.
Mitteroecker, P., Gunz, P., Windhager, S., Schaefer, K., 2013. A brief review of shape, form, and allometry in geometric morphometrics, with applications to human facial morphology. Hystrix 24, 59–66.
Mones, A., 1991. Monografía de la familia Hydrochoeridae (Mammalia: Rodentia). Senckenbergische Naturforschende Gesellschaft 134, 1–235.
Mones, A., Ojasti, J., 1986. Hydrochoerus hydrochaeris. Mamm. Species, 1–7.
Moreira, J.R., Alvarez, M.R., Tarifa, T., Pacheco, V., Taber, A., Tirira, D.G., Herrera, E.A., Ferraz, K.M.P.M.B., Aldana-Domínguez, J., Macdonald, D.W., 2013. Taxonomy, natural history and distribution of the capybara. In: Moreira, J.R., Ferraz, K.M.P.M.B., Herrera, E.A., Macdonald, D.W. (Eds.), Capybara: Biology, Use and Conservation of an Exceptional Neotropical Species. Springer Science & Business Media, pp. 3–37.
Morrone, J.J., 2014. Cladistic biogeography of the Neotropical region: identifying the main events in the diversification of the terrestrial biota. Cladistics 30, 202–214, https://doi.org/10.1111/cla.12039.
Nasif, N.L., Abdala, F., 1873. Craniodental ontogeny of the pacarana Dinomys branickii Peters (Rodentia, Hystricognathi, Caviomorpha, Dinomyidae). J. Mamm. 96, 1224–1244, https://doi.org/10.1093/jmammal/gyv131.
O’Regan, H.J., Kitchener, A.C., 2005. The effects of captivity on the morphology of captive, domesticated and feral mammals. Mamm. Rev. 35, 215–230, https://doi.org/10.1111/j.1365-2907.2005.00070.x.
Ojasti, J., 2011. Estudio Biológico Del Chigüire o Capibara. Equinoccio—Universidad Simón Bolívar—Academia De Ciencias Físicas, Matemáticas y Naturales, Caracas.
Ozgul, A., Childs, D.Z., Oli, M.K., Armitage, K.B., Blumstein, D.T., Olson, L.E., Tuljapurkar, S., Coulson, T., 2010. Coupled dynamics of body mass and population growth in response to environmental change. Nature 466 (7305), 482–485, https://doi.org/10.1038/nature09210.
Pérez, M.E., Vallejo-Pareja, M.C, Carrillo, J.D. Jaramillo, C., 2016. Pliocene capybaras (Rodentia, Caviidae) from northern South America (Guajira, Colombia), and its implications in the Great American Biotic Interchange. J. Mamm. Evol. R Core Team, 2015. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria https://doi.org/www.R-project.org//.
Rohlf, F.J., Bookstein, F.L., 2003. Computing the uniform component of shape variation. Syst. Biol. 52, 66–69, https://doi.org/10.1080/10635150390132759.
Rohlf, F.J., Slice, D., 1990. Extensions ofthe Procrustes method forthe optimal superimposition of landmarks. Syst. Zool. 39, 40–59.
Saragusty, J., Shavit-Meyrav, A., Yamaguchi, N., Nadler, R., Bdolah-Abram, T., Gibeon, L., Hildebrandt, T.B., Shamir, M.H., 2014. Comparative skull analysis suggests species-specific captivity-related malformation in lions (Panthera leo). PloS one 9, https://doi.org/10.1371/journal.pone.0094527.
Segura, V., Prevosti, F., 2012. A quantitative approach to the cranial ontogeny of Lycalopex culpaeus (Carnivora: Canidae). Zoomorphology 131, 79–92, https://doi.org/10.1007/s00435-012-0145-4.
Segura, V., Prevosti, F., Cassini, G., 2013. Cranial ontogeny in the puma lineage, puma concolor Herpailurus yagouaroundi, and Acinonyxjubatus (carnivora: Felidae): a three-dimensional geometric morphometric approach. Zool. J. Linn. Soc. 169, 235–250, https://doi.org/10.1111/zoj.12047.
Sheets, H.D., Zelditch, M.L., 2013. Studying ontogenetic trajectories using resampling methods and landmark data. Hystrix Italian J. Mamm. 24, 67–73 https://doi.org/10.4404/hystrix-24.1-6332.
Swiderski, D.L., Zelditch, M.L., 2013. The complex ontogenetic trajectory of mandibular shape in a laboratory mouse. J. Anat. 223, 568–580, https://doi.org/10.1111/joa.12118.
Tanner, J.B., Zelditch, M.L., Lundrigan, B.L., Holekamp, K.E., 2010. Ontogenetic change in skull morphology and mechanical advantage in the spotted hyena (Crocuta crocuta). J. Morphol. 271, 353–365, https://doi.org/10.1002/jmor.10802.
Trapido, H., 1949. Gestation period, young and maximum weight ofthe Isthmian capybara. Hydrochoerus isthmius Goldman. J. Mamm. 30, 433, https://doi.org/10.1093/jmammal/30.4.433.
Valenzuela-Lamas, S., Baylac, M., Cucchi, T., Vigne, J.D., 2011. House mouse dispersal in Iron Age Spain : a geometric morphometrics appraisal. Biol. J. Linn. Soc. 102, 483–497, https://doi.org/10.1111/j.1095-8312.2010.01603.x.
Vassallo, A.I., Antenucci, D.A., 2015. Biology of caviomorph rodents: diversity and evolution. In: SAREM Series A Mammalogical Research. Buenos Aires.
Vucetich, M.G., Deschamps, CM., Olivares, A.I., Dozo, M.T., 2005. Capybaras, size, shape, and time: a model kit. Acta Palaeontol. Polonica 50, 259–272.
Vucetich, M.G., Deschamps, CM., Pérez, M.E., Montalvo, C.I., 2014. The taxonomic status ofthe Pliocene capybaras (Rodentia) Phugatherium Ameghino and Chapalmatherium Ameghino. Ameghiniana 51, 173–183 dx.doi.org/10.5710/AMGH.05.02.2014.2074.
Vucetich, M.G., Deschamps, CM., Pérez, M.E., 2015a. The first capybaras (Rodentia, Caviidae Hydrochoerinae) involved in the Great American Biotic Interchange. Ameghiniana 52, 324–333, https://doi.org/10.5710/AMGH.07.10.2015.2891.
Vucetich, M.G., Arnal, M., Deschamps, CM., Pérez, M.E., Vieytes, E.C, 2015b. A brief history of caviomorph rodents as told by the fossil record. In: Vassallo, A.I., Antenucci, D. (Eds.), Biology of Caviomorph Rodents: Diversity and Evolution. SAREM Series A Mammalogical Research., pp. 11–62.
Weisbecker, V., Schmid, S., 2008. Autopodial skeletal diversity in hystricognath rodents: functional and phylogenetic aspects. Mamm. Biol. 72 (1), 27–44.
Weston, E.M., 2003. Evolution of ontogeny in the hippopotamus skull: using allometry to dissect developmental change. Biol. J. Linn. Soc. 80, 625–638.
Wilson, L.A.B., 2011. Comparison of prenatal and postnatal ontogeny: cranial allometry in the African striped mouse (Rhabdomys pumilio). J. Mamm. 92, 407–420.
Wilson, L.A.B., Werneburg, I., 2014. Quantifying evolutionary development using non-model organisms: integrating morphology, metrical frameworks, and gene expression. J. Exp. Zool. Part B: Mol. Dev. Evol. 322 (8), 555–557.
Zelditch, M.L., Swiderski, D.L., Sheets, H.D., Fink, W.L., 2004a. Geometric Morphometrics for Biologists: a Primer. Elsevier Academic Press, San Diego.
Zelditch, M.L., Lundrigan, B., Sheets, H.D., Garland, T., 2003. Do precocial mammals develop at a faster rate? A comparison of rates of skull development in Sigmodon fulviventer and Mus musculus domesticus. J. Evol. Biol. 16 (4), 708–720, https://doi.org/10.1046/j.1420-9101.2003.00568.x.
Zelditch, M.L., Lundrigan, B., Garland, T., 2004b. Developmental regulation of skull morphology. I. Ontogenetic dynamics of variance. Evol. Dev. 6 (3), 194–206, https://doi.org/10.1111/j.1525-142X.2004.04025.x.
Zelditch, M.L., Calamari, Z.T., Swiderski, D.L., 2016. Disparate postnatal ontogenies do not add to the shape disparity of infants. Evol. Biol., https://doi.org/10.1007/s11692-016-9370-y.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aeschbach, M., Carrillo, J.D. & Sánchez-Villagra, M.R. On the growth of the largest living rodent: Postnatal skull and dental shape changes in capybara species (Hydrochoerus spp.). Mamm Biol 81, 558–570 (2016). https://doi.org/10.1016/j.mambio.2016.02.010
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.mambio.2016.02.010