Abstract
Objective
The cell membrane water channel protein aquaporins (AQPs) may be important in regulating the intramembranous (IM) pathway of amnio tic fluid (AF) resorption. The objective of the present study was to determine whether aquaporin 3 (AQP3) is expressed in human fetal membranes and to further determine if A QP3 expression in primary human amnion cell culture is regulated by second-messenger cyclic adenosine monophosphate (cAMP)
Methods
AQP3 expression in human fetal membranes of normal term pregnancy was studied by reverse transcription polymerase chain reaction (RT-PCR) and immunohistochemistry (IHC). To determine the effect of cAMP on AQP3 expression, primary human amnion cell cultures were treated in either heat-inactivated medium alone (control), or heat-inactivated medium containing: (1) SP-cAMP, a membrane-permeable and phosphodiesterase resistant cAMP agonist, or (2) forskolin, an adenylate cyclase stimulator. Total RNA was isolated and multiplex real-time RT-PCR employed for relative quantitation of A QP3 expression.
Results
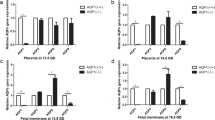
We detected AQP3 expression in placenta, chorion, and amnion using RT-PCR. Using IHC, we identified AQP3 protein expression in placenta syncytio trop hob las ts and cytotrophoblasts, chorion cytotrophoblasts, and amnion epithelia. In primary amnion epithelial cell culture, AQP3 mRNA signifi-cantly increased at 2 hours following forskolin or SP-cAMP, remained elevated at 10 hours following forskolin, and returned to baseline levels by 20 hours following treatment.
Conclusion
This study provides evidence of AQP3 expression in human fetal membranes and demonstrates that AQP3 expression in primary human amnion cell culture is up-regulated by second- messenger cAMP. As AQP3 is permeable to water, urea, and glycerol, modulation of its expression in fetal membranes may contribute to AF homeostasis.
Similar content being viewed by others
References
Knepper MA, Wade JB, Terris J, et al. Renal aquaporins. Kidney Int 1996;49:1712–7.
Ishibashi K, Sasaki S, Fushimi K, et al. Molecular cloning and expression of a member of the aquaporin family with permeability ot glycerol and urea in addition to water expressed at the basolateral membrane of kidney collecting duct cells. Proc Natl Acad Sci U S A 1994;91:6269–73.
Hamann S, Zeuthen T, La Cour M, et al. Aquaporins in complex tissues: Distribution of aquaporins 1-5 in human and rat eye. Am J Physiol 1998;274:C1332–45.
Underwood MA, Gilbert WM, Sherman MP. Amniotic fluid: Not just fetal urine anymore. J Perinatol 2005;25:341–8.
Hardy MA, Leonardi RT, Scheide JI. Cellular permeation pathways in a leaky epithelium: The human amniochorion. Biol Cell 1989;66:149–53.
Brace RA, Vermin ML, Huijssoon E. Regulation of amniotic fluid volume: Intramembranous solute and volume fluxes in late gestation fetal sheep. Am J Obstet Gynecol 2004;191:837–46.
Gilbert WM, Brace RA. The missing link in amniotic fluid volume regulation: Intramembranous absorption. Obstet Gynecol 1989;74:748–54.
Lingwood B, Wintour EM. Amniotic fluid volume and in vivo permeability of ovine fetal membranes. Obstet Gynecol 1984;64:368–72.
Gilbert WM, Newman PS, Eby-Wilkens E, Brace RA. Technetium Tc 99m rapidly crosses the ovine placenta and intramembranous pathway. Am J Obstet Gynecol 1996;175:1557–62.
Matsuzaki T, Suzuki T, Takata K. Hypertonicity-induced expression of aquaporin 3 in MDCK cells. Am J Physiol Cell Physiol 2001;281:C55–63.
Li C, Wang W, Kwon TH, et al. Downregulation of AQP1, -2, and -3 after ureteral obstruction is associated with a long-term urine-concentrating defect. Am J Physiol Renal Physiol 2001;281:F163–71.
Itoh A, Tsujikawa T, Fujiyama Y, Bamba T. Enhancement of aquaporin-3 by vasoactive intestinal polypeptide in a human colonic epithelial cell line. J Gastroenterol Hepatol 2003;18:203–10.
Johnston H, Koukoulas I, Jeyaseelan K, et al. Ontogeny of aquaporins 1 and 3 in ovine placenta and fetal membranes. Placenta 2000;21:88–99.
Wlodek ME, Challis JRG, Patrick J. Urethral and urachal urine output to the amniotic and allantoic sacs in fetal sheep. J Dev Physiol 1988;10:309–19.
Harding R, Bocking AD, Sigger JN, Wickham PJ. Composition and volume of fluid swallowed by fetal sheep. Q J Exp Physiol 1984;69:487–95.
Preston GM, Carroll TP, Guggino WB, Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 1992;256:385–7.
Wang S, Kallichanda N, Song W, Ramierez B, Ross MG. Expression of aquaporin 8 in human placenta and chorioamniotic membranes: Evidence of molecular mechanism for intramembranous amniotic fluid resorption. Am J Obstet Gynecol 2001;185:1226–31.
Wang S, Chen J, Beall M, Zhou W, Ross MG. Expression of aquaporin 9 in human chorioamniotic membranes and placenta. Am J Obstet Gynecol 2004;191:2160–7.
Mann SE, Ricke EA, Yang BA, Verkman AS, Taylor RN. Expression and localization of aquaporin 1 and 3 in human fetal membranes. Am J Obstet Gynecol 2002;187:902–7.
Wang S, Chen J, Au KT, Ross MG. Expression of aquaporin 8 and its up-regulation by cyclic adenosine monophosphate in human WISH cells. Am J Obstet Gynecol 2003;188:997–1001.
Ross MG, Wang S. Wishing the WISH cells were pure. Am J Obstet Gynecol 2003;189:1807–8.
Lee DW, Markoff E. Synthesis and release of glycosylated prolactin by human decidua in vitro. J Clin Endocrinol Metab 1986;62:990–4.
Maaskant RA, Bogic LV, Gilger S, Kelly PA, Bryant-Greenwood GD. The human prolactin receptor in the fetal membranes, decidua, and placenta. J Clin Endocrinol Metab 1996;81:396–405.
Bakker-Teunissen OJ, Arts NF, Mulder GH. Fluid transport across human fetal membranes affected by human amniotic fluid prolactin: An in vitro study. Placenta 1988;9:533–45.
Vizsolyi E, Perks AM. The effect of arginine vasotocin on the isolated amniotic membrane of the guinea pig. Can J Zoology 1974;52:371–86.
Benedetto MT, De Cicco F, Rossiello F, Nicosia AL, Lupi G, Dell’Acqua S. Oxytocin receptor in human fetal membranes at term and during labor. J Steroid Biochem 1990;35:205–8.
Phillippe M, Ryan KJ. Catecholamines in human amniotic fluid. Am J Obstet Gynecol 1981;139:204–8.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by National Institutes of Health Grants No. HL 40899, HD 044482, RR 00425, and the March of Dimes Birth Defect Foundation.
Rights and permissions
About this article
Cite this article
Wang, S., Amidi, F., Beall, M. et al. Aquaporin 3 Expression in Human Fetal Membranes and its Up-regulation by Cyclic Adenosine Monophosphate in Amnion Epithelial Cel Culture. Reprod. Sci. 13, 181–185 (2006). https://doi.org/10.1016/j.jsgi.2006.02.002
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.jsgi.2006.02.002