Abstract
Objective
The aim of the present study was to evaluate a rat model of placental dysfunction/ preeclampsia in pregnancies complicated by maternal diabetes. A second objective was to evaluate the effects of vitamin E treatment in this model.
Methods
Normal and streptozotocin-induced diabetic rats of two different strains (U and H) were given intraperitoneal (IP) injections of the angiogenesis inhibitor Suramin (Sigma Chemical Co, St Louis, MO) or saline in early pregnancy, and fed standard or vitamin E— enriched food. The outcome of pregnancy was evaluated on gestational day 20.
Results
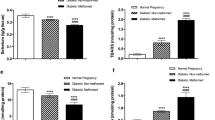
In both rat strains Suramin caused fetal growth retardation, decreased placental blood flow, and increased placental concentration of the isoprostane 8-iso-PGF2α. In the U rats Suramin also caused increased fetal resorption rate, increased maternal blood pressure, decreased renal blood flow, and diminished maternal growth. Diabetes caused severe maternal and fetal growth retardation, increased resorption rate, and increased placental 8-iso-PGF2α concentration independent of Suramin administration. The maternal and fetal effects of Suramin and diabetes were more pronounced in the U strain than in the H strain. Vitamin E treatment improved the status of Suramin-injected diabetic rats: in U rats the blood pressure increase was normalized; and in both U and H rats the decreased placental blood flow was marginally enhanced, and the increase in placental 8-iso-PGF2α was partly normalized by vitamin E.
Conclusion
Suramin injections to pregnant rats cause a state of placental insufficiency, which in U rats resembles human preeclampsia. The induction of this condition is at least partly mediated by oxidative stress, and antagonized by antioxidative treatment. Maternal diabetes involves increased oxidative stress, and causes both maternal and fetal morbidity, which are only marginally affected by additional Suramin treatment.
Similar content being viewed by others
References
Chesley LC, Duffus GM. Preeclampsia, posture and renal function. Obstet Gynecol 1971;38:1–5.
Hubel CA, Roberts JM, Taylor RN, Musci TJ, Rogers GM, McLaughlin MK. Lipid peroxidation in pregnancy: New perspectives on preeclampsia. Am J Obstet Gynecol 1989;161:1025–34.
Dekker GA, Sibai BM. Etiology and pathogenesis of preeclampsia: current concepts. Am J Obstet Gynecol 1998;179:1359–75.
Hung TH, Skepper JN, Burton GJ. In vitro ischemia-reperfusion injury in term human placenta as a model for oxidative stress in pathological pregnancies. Am J Pathol 2001;159:1031–43.
Redman CW, Sargent IL. The pathogenesis of pre-eclampsia. Gynecol Obstet Fertil 2001;29:518–22.
Kauma SW, Wang Y, Walsh SW. Preeclampsia is associated with decreased placental interleukin-6 production. J Soc Gynecol Investig 1995;2:614–7.
Caniggia I, Winter J, Lye SJ, Post M. Oxygen and placental development during the first trimester: Implications for the pathophysiology of pre-eclampsia. Placenta 2000;21(suppl):S25–30.
Arngrimsson R, Bjornsson S, Geirsson RT, Bjornsson H, Walker JJ, Snaedal G. Genetic and familial predisposition to eclampsia and pre-eclampsia in a defined population. Br J Obstet Gynaecol 1990;97:762–9.
Yamada N, Arinami T, Yamakawa-Kobayashi K, et al. The 4G/5G polymorphism of the plasminogen activator inhibitor-1 gene is associated with severe preeclampsia. J Hum Genet 2000;45:138–41.
Treloar SA, Cooper DW, Brennecke SP, Grehan MM, Martin NG. An Australian twin study of the genetic basis of preeclampsia and eclampsia. Am J Obstet Gynecol 2001;184:374–81.
Wang Y, Walsh S. Antioxidant activities and mRNA expression of superoxide dismutase, catalase, and glutathione peroxidase in normal and preeclamptic placentas. J Soc Gynecol Investig 1996;3:179–84.
Hubel CA. Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med 1999;222:222–35.
Akyol D, Mungan T, Gorkemli H, Nuhoglu G. Maternal levels of vitamin E in normal and preeclamptic pregnancy. Arch Gynecol Obstet 2000;263:151–5.
Many A, Hubel CA, Fisher SJ, Roberts JM, Zhou Y. Invasive cytotrophoblasts manifest evidence of oxidative stress in preeclampsia. Am J Pathol 2000;156:321–31.
Walsh SW, Vaughan JE, Wang Y, Roberts LJ 2nd. Placental isoprostane is significantly increased in preeclampsia. FASEB J 2000;14:1289–96.
Staff AC, Ranheim T, Henriksen T, Halvorsen B. 8-Iso-prostaglandin f(2alpha) reduces trophoblast invasion and matrix metalloproteinase activity. Hypertension 2000;35:1307–13.
Raijmakers MT, Zusterzeel PL, Roes EM, Steegers EA, Mulder TP, Peters WH. Oxidized and free whole blood thiols in preeclampsia. Obstet Gynecol 2001;97:272–6.
Takacs P, Kauma SW, Sholley MM, Walsh SW, Dinsmoor MJ, Green K. Increased circulating lipid peroxides in severe preeclampsia activate NF-kappaB and upregulate ICAM-1 in vascular endothelial cells. FASEB J 2001;15:279–81.
Chappell LC, Seed PT, Briley AL, et al. Effect of antioxidants on the occurrence of pre-eclampsia in women at increased risk: A randomized trial. Lancet 1999;354:810–6.
Chappell LC, Seed PT, Kelly FJ, et al. Vitamin C and E supplementation in women at risk of preeclampsia is associated with changes in indices of oxidative stress and placental function. Am J Obstet Gynecol 2002;187:777–84.
Combs CA, Rosenn B, Kitzmiller JL, Khoury JC, Wheeler BC, Miodovnik M. Early-pregnancy proteinuria in diabetes related to preeclampsia. Obstet Gynecol 1993;82:802–7.
Hsu CD, Tan HY, Hong SF, Nickless NA, Copel JA. Strategies for reducing the frequency of preeclampsia in pregnancies with insulin-dependent diabetes mellitus. Am J Perinatol 1996;13:265–8.
Hanson U, Persson B. Epidemiology of pregnancy-induced hypertension and preeclampsia in type 1 (insulin-dependent) diabetic pregnancies in Sweden. Acta Obstet Gynecol Scand 1998;77:620–4.
Ros HS, Cnattingius S, Lipworth L. Comparison of risk factors for preeclampsia and gestational hypertension in a population-based cohort study. Am J Epidemiol 1998;147:1062–70.
Hiilesmaa V, Suhonen L, Teramo K. Glycaemic control is associated with pre-eclampsia but not with pregnancy-induced hypertension in women with type I diabetes mellitus. Diabetologia 2000;43:1534–9.
West IC. Radicals and oxidative stress in diabetes. Diabet Med 2000;17:171–80.
Aydin A, Orhan H, Sayal A, Ozata M, Sahin G, Isimer A. Oxidative stress and nitric oxide related parameters in type II diabetes mellitus: Effects of glycemic control. Clin Biochem 2001;34:65–70.
Sheetz MJ, King GL. Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA 2002;288:2579–88.
Ishihara G, Hiramatsu Y, Masuyama H, Kudo T. Streptozotocin-induced diabetic pregnant rats exhibit signs and symptoms mimicking preeclampsia. Metabolism 2000;49:853–7.
Eriksson UJ, Jansson L. Diabetes in pregnancy: decreased placental blood flow and disturbed fetal development in the rat. Pediatr Res 1984;18:735–8.
Nash P, Wentzel P, Lindeberg S, et al. Placental dysfunction in Suramin-treated rats—A new model for pre-eclampsia. Placenta 2005 (in press).
Eriksson UJ. Importance of genetic predisposition and maternal environment for the occurrence of congenital malformations in offspring of diabetic rats. Teratology 1988;37:365–74.
Eriksson UJ, Andersson A, Efendic S, Elde R, Hellerström C. Diabetes in pregnancy: effects on the fetal and newborn rat with particular regard to body weight, serum insulin concentration and pancreatic contents of insulin, glucagon and somatostatin. Acta Endocrinol (Copenh) 1980;94:354–64.
Lowry OH, Rosebrough. NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265–75.
Allain CC, Poon LS, Chon CSG, Richmond U, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem 1974;20:470–5.
Bondar RJL, Mead DC. Evaluation of glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides in the hexokinase method for determining glucose in serum. Clin Chem 1974;20:586–90.
Maas AH. IFCC reference methods and materials for measurement of pH, gases and electrolytes in blood. Scand J Clin Lab Invest 1993;214:83–94.
Doumas BT. Standards for total serum protein assays-A collaborative study. Clin Chem 1975;21:1159–66.
Jacobs NJ, Vandemark PJ. The purification and properties of the alpha-glycerophosphate-oxidizing enzyme of Streptococcus faecalis 10C1. Arch Biochem Biophys 1960;88:250–5.
Moshage H, Kok B, Huizenga JR, Jansen PL. Nitrite and nitrate determinations in plasma: A critical evaluation. Clin Chem 1995;41:892–6.
Pradelles P, Grassi J, Maclouf J. Enzyme immunoassays of eicosanoids using acetylcholine esterase as label: An alternative to radioimmunoassay. Anal Chem 1985;57:1170–3.
Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Med Sci 1959;37:911–7.
Morrow JD, Harris TM, Roberts LJ 2nd. Noncyclooxygenase oxidative formation of a series of novel prostaglandins: Analytical ramifications for measurement of eicosanoids. Anal Biochem 1990;184:1–10.
Svensson AM, Bodin B, Andersson A, Jansson L. Pancreatic islet blood flow during pregnancy in the rat: An increased islet mass is associated with decreased islet blood flow. J Endocrinol 2004;180:409–15.
Gagliardi A, Hadd H, Collins DC. Inhibition of angiogenesis by suramin. Cancer Res 1992;52:5073–5.
Bocci G, Danesi R, Benelli U, et al. Inhibitory effect of suramin in rat models of angiogenesis in vitro and in vivo. Cancer Chemother Pharmacol 1999;43:205–12.
Zygmunt M, Herr F, Münstedt K, Lang U, Liang OD. Angiogenesis and vasculogenesis in pregnancy. Eur J Obstet Gynecol Reprod Biol 2003;110 Suppl 1: S10–8.
Bishop ET, Bell GT, Bloor S, Broom IJ, Hendry NF, Wheatley DN. An in vitro model of angiogenesis: Basic features. Angiogenesis 1999;3:335–44.
Freeman SJ, Lloyd JB. Evidence that suramin and aurothiomalate are teratogenic in rat by disturbing yolk sac-mediated embryonic protein nutrition. Chem Biol Interact 1986;58:149–60.
Lloyd JB, Beck F. Lysosomes and congenital malformations. Biochem J 1969;115:32P–4P.
Lee CS, Han JH, Lee SM, et al. Wax moth, Galleria mellonella fat body receptor for high-density lipophorin (HDLp). Arch Insect Biochem Physiol 2003;54:14–24.
Tannert A, Wustner D, Bechstein J, Muller P, Devaux PF, Herrmann A. Aminophospholipids have no access to the luminal side of the biliary canaliculus: Implications for the specific lipid composition of the bile fluid. J Biol Chem 2003;278:40631–9.
Eriksson UJ, Dahlstrom VE, Lithell HO. Diabetes and pregnancy: Influence of genetic background and maternal diabetic state on the incidence of skeletal malformations in the fetal rat. Acta Endocrinol (Copenh) 1986;112 Suppl 277:66–73.
Eriksson UJ, Styrud J, Eriksson RSM. Diabetes in pregnancy: Genetic and temporal relationships of maldevelopment in the offspring of diabetic rats. In: Sutherland HW, Stowers JM, Pearson DWM, eds. 4th International Colloquium on Carbohydrate Metabolism in Pregnancy and the Newborn. Berlin: Springer-Verlag, 1989:51–63.
Styrud J, Thunberg L, Nybacka O, Eriksson UJ. Correlations between maternal metabolism and deranged development in the offspring of normal and diabetic rats. Pediatr Res 1995;37:343–53.
Wood SM. Assessment of renal functions in hypertensive pregnancies. Clin Obstet Gynaecol 1977;4:747–58.
Kublickas M, Lunell NO, Nisell H, Westgren M. Maternal renal artery blood flow velocimetry in normal and hypertensive pregnancies. Acta Obstet Gynecol Scand 1996;75:715–9.
Klein T, Neuhaus K, Reutter F, Nusing RM. Generation of 8-epi-prostaglandin F(2alpha) in isolated rat kidney glomeruli by a radical-independent mechanism. Br J Pharmacol 2001;133:643–50.
Cederberg J, Basu S, Eriksson UJ. Increased rate of lipid peroxidation and protein carbonylation in experimental diabetic pregnancy. Diabetologia 2001;44:766–74.
Wentzel P, Welsh N, Eriksson UJ. Developmental damage, increased lipid peroxidation, diminished cyclooxygenase-2 gene expression, and lowered PGE2 levels in rat embryos exposed to a diabetic environment. Diabetes 1999;48:813–20.
Eriksson UJ, Borg LAH. Protection by free oxygen radical scavenging enzymes against glucose-induced embryonic malformations in vitro. Diabetologia 1991;34:325–31.
Simán CM, Gittenberger-De Groot AC, Wisse B, Eriksson UJ. Malformations in offspring of diabetic rats: Morphometric analysis of neural crest-derived organs and effects of maternal vitamin E treatment. Teratology 2000;61:355–67.
Simán CM, Eriksson UJ. Vitamin E decreases the occurrence of malformations in the offspring of diabetic rats. Diabetes 1997;46:1054–61.
Cederberg J, Siman CM, Eriksson UJ. Combined treatment with vitamin E and vitamin C decreases oxidative stress and improves fetal outcome in experimental diabetic pregnancy. Pediatr Res 2001;49:755–62.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Ernfors Family Fund, Swedish Diabetes Association, Novo Nordisk Foundation, Swedish Research Council (Grants No. 12X-7475, 12X-109, 73X-08683-16A), General Maternity Hospital Foundation, Magnus Bergvall’s Foundation, and Henning and Gosta Ankarstrand’s Foundation.
The authors are grateful to Lisbeth Sagulin for practical help during the investigation, and to Dr Parri Wentzel and Dr Leif Jansson for valuable comments and substantial support.
Rights and permissions
About this article
Cite this article
Nash, P., Olovsson, M. & Eriksson, U.J. Placental Dysfunction in Suramin-Treated Rats: Impact of Maternal Diabetes and Effects of Antioxidative Treatment. Reprod. Sci. 12, 174–184 (2005). https://doi.org/10.1016/j.jsgi.2004.12.002
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.jsgi.2004.12.002