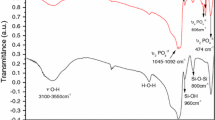
Abstract
The purpose of this study is to explore and develop a novel biocompatibility drug delivery carrier for controllingontrolled drug release. The α-eleostearic acid grafted hydroxyapatite (α-ESA-g-HA) composite was synthesized by using silane coupling agent and characterized by Fourier Transformation Infrared Spectroscopy (FT-IR), Thermal Gravimetric Analysis (TGA) and Scanning Electron Microscope (SEM), respectively. The in vitro drug loading and controlled release behaviors of α-ESA-g-HA composite were investigated using ciprofloxacin as the model drug. The amount of ciprofloxacin loading and released was calculated by absorbance value which was determined by UV-Vis spectrophotometry at wavelength of 277 nm. The biocompatibility of α-ESA-g-HA composite was assessed by 3-(4,5)-dimethylthiahiazo(-z-yl)-3,5-di-phenytetrazoliumromide(MTT) assay, nuclear morphology and platelet adhesion. The results showed that the α-ESA-g-HA had nontoxic and good biocompatibility. According to the results mentioned above, the α-ESA-g-HA is an effective drug delivery carrier, which could increase drug loading capacity and control drug release, so further studies are necessary to evaluate clinical application and human health care.
Similar content being viewed by others
References
Langer R. New methods of drug delivery. Science, 1990, 249, 1527–1533.
Huang Y C, Lam U I. Chitosan/fucoidan pH sensitive nanoparticles for oral delivery system. Journal of the Chinese Chemical Society, 2011, 58, 779–785.
Slowing I I, Vivero-Escoto J L, Wu C W, Lin V S. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Advanced Drug Delivery Reviews, 2008, 60, 1278–1288.
Xiao B L, Ma Z Y, Bl J. Creep behavior of TiBw/Ti and (TiBw + TiCp)/Ti in situ composite. Journal of Materials Science Letters, 2002, 21, 859–861.
Peter M, Ganesh N, Selvamurugan N, Nair S V, Furuike T, Tamura H, Jayakumar R. Preparation and characterization of chitosangelatin/nanohydroxyapatite composite scaffolds for tissue engineering applications. Carbohydrate Polymers, 2010, 80, 687–694.
Nie H, Wang C H. Fabrication and characterization of PLGA/HAp composite scaffolds for delivery of BMP-2 plasmid DNA. Journal of controlled release, 2007, 120, 111–121.
Saleem I Y, Vordermeier M, Barralet J E, Coombes A G. Improving peptide-based assays to differentiate between vaccination and Mycobacterium bovis infection in cattle using nanoparticle carriers for adsorbed antigens. Journal of controlled release, 2005, 102, 551–561.
Matsumoto T, Okazaki M, Inoue M, Yamaguchi S, Kusun-ose T, Toyonaga T, Hamada Y, Takahashi J. Hydroxyapatite particles as a controlled release carrier of protein. Biomaterials, 2004, 25, 3807–3812.
Sibilla P, Sereni A, Aguiari G, Banzi M, Manzati E, Mischiati C, Trombelli L, del Senno L. Effects of a hydroxyapa-tite-based biomaterial on gene expression in osteoblast-like cells. Journal of Dental Research, 2006, 85, 354–358.
Dasgupta S, Bandyopadhyay A, Bose S. Reverse micelle-mediated synthesis of calcium phosphate nanocarriers for release of bovine serum albumin. Acta Biomaterialia, 2009, 5, 3112–3121.
Liu T Y, Chen S Y, Liu D M, Liou S C. On the study of BSA-loaded calcium-deficient hydroxyapatite nano-carriers for controlled drug delivery. Journal of Controlled Release, 2005, 107,112–121.
Palazzo B, Iafisco M, Laforgia M, Margiotta N, Natile G, Bianchi C L. Biomimetic hydroxyapatite-drug nanocrystals as potential bone substitutes with antitumor drug delivery properties. Advanced Functional Materials, 2007, 17, 2180–2188.
Zhu X, Eibl O, Scheideler L, Geis-Gerstorfer J. Characterization of nano hydroxyapatite/collagen surfaces cellular behaviors. Journal Biomedical Materials Reseach A, 2006, 79, 114–127.
Zhu X L, Eibl O, Berthold C, Scheideler, L, Geis-Gerstorfer. Some fundamental aspects of mechanics of nanocomposite materials and structural members. Journal of Nanotechnol-ogy, 2006, 17, 2711–2721.
Cai Y, Liu Y, Yan W, Hu Q, Tao J, Zhang M, Shi Z, Tang R. Role of hydroxyapatite nanoparticle size in bone cell proliferation. Journal of Materials Chemistry, 2007, 17, 3780–3787.
Y Mizushima, T Ikoma, J Tanaka, K Hoshi, T Ishihara, Y Ogawa, A Ueno. Injectable porous hydroxyapatite microparticles as a new carrier for protein and lipophilic drugs. Journal of Controlled Release, 2006, 110, 260–265.
Liu Q, De Wijn J R, Bakker D, Van Blitterswijk C A. Surface modification of hydroxyapatite to introduce interfacial bonding with polyactiveTM 70/30 in a biodegradable composite. Journal of Materials science: Materials in Medicine, 1996, 7, 551–557.
Murugan R, Ramakrishna S. Coupling of therapeutic molecules onto surface modified coralline hydroxyapatite. Biomaterials, 2004, 25, 3073–3080.
D’Andrea S C, Fadeev A Y. Covalent surface modification of calcium hydroxyapatite using n-Alkyl- and n-Fluoroalky-lphosphonic acids. Langmuir, 2003, 19, 7904–7910.
Borum-Nicholas L, Wilson Jr O C. Surface modification hydroxyapatite. Part I. Dodecyl alcohol. Biomaterials, 2003, 24, 3671–3679.
Vega E D, Narda G E, Ferretti F H. Adsorption of citric acid from dilute aqueous solutions by hydroxyapatite. Journal of Colloid and Interface Science, 2003, 268, 37–42.
Borum L, Wilson Jr O C. Surface modification of hydroxyapatite. Part II. Silica. Biomaterials, 2003, 24, 3681–3688.
Liu Q, de Wijn J R, van Blitterswijk C A. Covalent bonding of PMMA, PBMA, and poly(HEMA) to hydroxyapatite particles. Journal of Biomedical Material Research, 1998, 40, 257–263.
Liu Q, de Wijn J R, de Groot K, van Blitterswijk C A. Surface modification of nano-apatite by grafting organic polymer. Biomaterials, 1998, 19, 1067–1072.
Theresa B, Price S L. Dimer or catemer? Lowenergy crystal packings for small carboxylic acids. Journal Physical Chemistry, 2000, 104, 2647–2655.
Eom J M, Seo M J, Baek J Y, Chu H, Han S H, Min T S, Cho C S, Yun C H. Alpha-eleostearic acid induces autophagy-dependent cell death through targeting AKT/mTOR and ERK1/2 signal together with the generation of reactive oxygen species. Biochemical and Biophysical Research Communications, 2010, 391, 903–908.
Zhou H, Lee J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomaterialia, 2011, 7, 2769–2781.
öner M, Yetiz E, Ay E. Ibuprofen release from porous hy-droxyapatite tablets. Ceramics International, 2011, 37, 2117–2125.
Cosijns A, Vervaet C, Luyten J, Mullens S, Siepmann F, Van Hoorebeke L, Masschaele B, Cnudde V, Remon J P. Porous hydroxyapatite tablets as carriers for low-dosed drugs. European Journal of Pharmaceutics and Biopharmaceutics, 2007, 67, 498–506.
Baradari H, Damia C, Dutreih-Colas M, Laborde E, Pecout N, Champion E, Chulia D, Viana M. Calcium phosphate porous pellets as drug delivery systems: Effect of drug carrier composition on drug loading and in vitro release. Journal of the European Ceramic Society, 2012, 32, 2679–2690.
öner M, Yetiz E, Ay E. Ibuprofen release from porous hy-droxyapatite tablets. Ceramics International, 2011, 37, 2117–2125.
Gbureck U, Vorndran E, Müller F A, Barralet J E. Low temperature direct 3D printed bioceramics and biocomposites as drug release matrices. Journal of Controlled Release, 2007, 122, 173–180.
Lu P, Fan H, Liu Y, Cao L, Wu X, Xu X. Controllable bio-degradability, drug release behavior and hemocompatibility of PTX-eluting magnesium stents. Colloids and Surfaces B: Biointerfaces, 2011, 83, 23–28.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cao, T., Tang, W., Zhao, J. et al. A Novel Drug Delivery Carrier Based on α-eleostearic Acid Grafted Hydroxyapatite Composite. J Bionic Eng 11, 125–133 (2014). https://doi.org/10.1016/S1672-6529(14)60027-5
Published:
Issue Date:
DOI: https://doi.org/10.1016/S1672-6529(14)60027-5