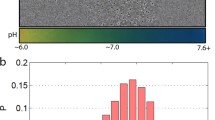
Abstract
Microrobots is playing more and more important roles for medical applications, such as targeting tumoral lesions for therapeutic purposes, Minimally Invasive Surgery (MIS) and highly localized drug delivery. However, energy efficient propulsion system poses significant challenges for the implementation of such mobile robots. Flagellated chemotactic bacteria can be used as an effective integrated propulsion system for microrobots. In this paper, we proposed a new type of propulsion method that is inspired by the motility mechanism of flagellated chemotactic bacteria in different pH gradients. The pH gradient field was established in solution through electrolysis method. The distribution of the pH values in solution was measured with pH indicator and analyzed with image processing technology, and the mechanism by which the pH values changed was also discussed. The swimming speed and direction of the bacteria were studied experimentally. Through analyzing the key parameters, such as stabilization time and electrode voltage, the optimal design of propulsion mechanism based on bacteria motion in the pH gradient field was proven.
Similar content being viewed by others
References
Behkam B, Sitti M. Modeling and testing of a biomimetic flagellar propulsion method for microscale biomedical swimming robots. Proceedings of the IEEE/ASME International Conference on Advanced Intelligent Mechatronics, Monterey, USA, 2005, 37–42.
Martel S, Mohammadi M, Felfoul O, Lu Z, Poupanneau P. Flagellated magnetotactic bacteria as controlled MRI-trackable propulsion and steering systems for medical nanorobots operating in the human microvasculature. International Journal of Robotics Research, 2009, 28, 571–581.
Julius A A, Sakar M S, Steager E, Cheang U K, Kim M J, Kumar V, Pappas G J. Harnessing bacterial powerin microscale zctuation. IEEE International Conference on Robotics and Automation, Kobe, Japan, 2009, 1004–1009.
Yu T S, Lauga E, Hosoi A E. Experimental investigations of elastic tail propulsion at low Reynolds number. Physics of Fluids, 2006, 18, 091071.
Purcell E M. Life at low Reynolds number. American Journal of Physics, 1977, 45, 3–11.
Steager E, Kim C, Patel J, Bith S, Naik C, Reber L, Kim M. Control of microfabricated structures powered by flagellated bacteria using phototaxis. Applied Physics Letters, 2007, 90, 263901.
Kim M, Breuer K. Use of bacterial carpets to enhance mixing in microfluidic systems. Journal of Fluids Engineering, 2007, 129, 319–324.
Behkam B, Sitti M. Bacterial flagella-based propulsion and on/off motion control of microscale objects. Applied Physics Letters, 2007, 90, 023902.
Behkamand B, Sitti M. Effect of quantity and configuration of attached bacteria on bacterial propulsion of microbeads. Applied Physics Letters, 2008, 93, 223901.
Ha N S, Goo N S. Propulsion modeling and analysis of a biomimetic swimmer. Journal of Bionic Engineering, 2010, 7, 259–266.
Yang C, Chen C, Ma Q, Wu L, Song T. Dynamic model and motion mechanism of magnetotactic bacteria with two lateral flagellar bundles. Journal of Bionic Engineering, 2012, 9, 200–210.
Sokolov A, Aranson I S, Kessler J O, Goldstein R E. Concentration dependence of the collective dynamics of swimming bacteria. Physisal Review Letters, 2007, 98, 158102.
Balagadd F K, You L, Hansen C L, Arnold F H, Quake S R. Long-term monitoring of bacteria undergoing programmed population control in a microchemostat. Science, 2005, 309, 137–140.
Keymer J E, Galajda P, Muldoon C, Park S, Austin R H. Bacterial metapopulations in nanofabricated landscapes. National Academy of Sciences, 2006, 103, 17290–17295.
Bard A J, Faulkner L R. Electrochemical Methods: Fundamentals and Applications. John Wiley and Sons, Inc., New York, USA, 1980.
Macnab R M. Examination of bacterial flagellation by dark-field microscopy. Journal of Clinical Microbiology, 1976, 4, 258–265.
Flores H, Lobaton E, Mendez-Diez S, Tlupova S, Cortez R. A study of bacterial flagellar bundling. Bulletin of Mathematical Biology, 2005, 67, 137–168.
Baker M D, Wolanin P M. Signal transduction in bacterial chemotaxis. BioEssays, 2005, 28, 9–22.
Berg H C, Brown D A. Chemotaxis in Escherichia coli analyzed by three-dimensional tracking. Nature, 1972, 239, 500–504.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, B., Wang, Z., Liu, C. et al. Swimming behavior analysis based on bacterial chemotaxis in solution. J Bionic Eng 9, 315–321 (2012). https://doi.org/10.1016/S1672-6529(11)60131-5
Published:
Issue Date:
DOI: https://doi.org/10.1016/S1672-6529(11)60131-5