Abstract
Background
We tested the hypotheses that (1) maternal betamethasone ($M) treatment over the range of clinical doses for prevention of prematurity-related pathologies from day 15 to 21 of rat gestation would produce growth retardation, and (2) the lowest βM dose to produce growth retardation would result in hypertension in adult offspring.
Methods
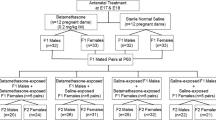
In experiment 1, pregnant Sprague-Dawley rats were administered βM (0, 50, 100, 200, 400, or 600 μg/kg per day, subcutaneously) on days 15–21 of pregnancy and necropsied on day 21.5, with fetal lung and placental weights recorded. In experiment 2, two more groups of rats (0 or 100 μg/kg per day, subcutaneously) were allowed to deliver, and offspring were instrumented at 100 ± 4 days of postnatal life with indwelling left carotid arterial catheters. After 48 hours of recovery, blood pressure was recorded continuously for 24 hours.
Results
In experiment 1, all newborn rats treated with βM, and their placentas, except those receiving 50 μg/kg per day, were growth retarded in comparison with controls (P <.05). All treated lungs were smaller than those of controls (P <.05). In experiment 2, no differences were found in the mean arterial blood pressure of adult offspring given the lowest effective dose βM (100 μg/kg per day) compared with controls (114.2 ± 5.3 mmHg versus 114.6 ± 3.4 mmHg, respectively).
Conclusion
These data suggest that glucocorticoids given in the last week of rat pregnancy in the lowest human clinical dose do not cause hypertension and somatic growth retardation. However, the presence of lung growth restriction at this dose argues for more studies on the efficacy of even lower concentrations for their ability to improve lung and other organ and tissue function while avoiding unwanted side effects.
Similar content being viewed by others
References
Barker DJP. Mothers, babies and health in later life. 2nd ed. New York: Churchill Livingstone, 1998.
Ashton N. Perinatal development and adult blood pressure. Braz J Med Biol Res 2000;33:731–40.
Barker DJ, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. BMJ 1990;301:259–62.
Barker DJP. Fetal origins of coronary heart disease. BMJ 1995;311:171–4.
Barker DJP, Osmond C, Golding J, Kuh D, Wadsworth MEJ. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ 1989;298:564–7.
Langley SC, Jackson AA. Increased systolic blood pressure in adult rats induced by fetal exposure to maternal low protein diets. Clin Sci (Colch) 1994;86:217–22.
Nwagwu MO, Cook A, Langley-Evans SC. Evidence of progressive deterioration of renal function in rats exposed to a maternal low-protein diet in utero. Br J Nutr 2000;83:79–85.
Benediktsson R, Lindsay RS, Noble J, Seckl JR, Edwards CRW. Glucocorticoid exposure in utero: New model for adult hypertension. Lancet 1993;341:339–41.
Lindsay RS, Lindsay RM, Edwards CR, Seckl JR. Inhibition of 11-beta-hydroxysteroid dehydrogenase in pregnant rats and the programming of blood pressure in the offspring. Hypertension 1996;27:1200–4.
Langley-Evans SC. Hypertension induced by foetal exposure to a maternal low-protein diet, in the rat, is prevented by pharmacological blockade of maternal glucocorticoid synthesis. J Hypertens 1997;15:537–44.
National Institutes of Health. The effect of antenatal steroids for fetal maturation on perinatal outcomes. NIH Consensus Statement 1994;12:1–24.
Levitt NS, Lindsay RS, Seckl JR. Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology 1996;64:412–8.
Doyle LW, Ford GW, Davis NM, Callanan C. Antenatal corticosteroid therapy and blood pressure at 14 years of age in preterm children. Clin Sci (Colch) 2000;98:137–42.
Celsi G, Kistner A, Aizman R, et al. Prenatal dexamethasone causes oligonephronia, sodium retention, and higher blood pressure in the offspring. Pediatr Res 1998;44:317–22.
Nelson W, Tong YL, Lee JK, Halberg F. Methods for cosinorrhythmometry. Chronobiologia 1979;6:305–23.
Waddell BJ, Hisheh S, Dharmarajan AM, Burton PJ. Apoptosis in rat placenta is zone-dependent and stimulated by glucocorticoids. Biol Reprod 2000;63:1913–7.
Sugden MC, Langdown ML, Munns MJ, Holness MJ. Maternal glucocorticoid treatment modulates placental leptin and leptin receptor expression and materno-fetal leptin physiology during late pregnancy, and elicits hypertension associated with hyperleptinaemia in the early-growth-retarded adult offspring. Eur J Endocrinol 2001;145:529–39.
Hardman JG, Limbird LE, eds. Goodman and Gilman’s the pharmacological basis of therapeutics. 9th ed. New York: McGraw-Hill, 1996.
Kovacs KJ, Makara GB. Corticosterone and dexamethasone act at different brain sites to inhibit adrenalectomy-induced adrenocorticotropin hypersecretion. Brain Res 1988;474:205–10.
Duke JL, Zammit TG, Lawson DM. The effects of routine cage-changing on cardiovascular and behavioral parameters in male Sprague-Dawley rats. Contemp Top Lab Anim Sci 2001;40:17–20.
Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension 2001;37:1199–208.
Manning J, Vehaskari VM. Low birth weight-associated adult hypertension in the rat. Pediatr Nephrol 2001;16:417–22.
Langley-Evans SC, Gardner DS, Jackson AA. Maternal protein restriction influences the programming of the rat hypothalamicpituitary-adrenal axis. J Nutr 1996;126:1578–85.
Kwong WY, Wild AE, Roberts P, Willis AC, Fleming TP. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development 2000;127:4195–202.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Institutes of Health grant HL65399.
Rights and permissions
About this article
Cite this article
McDonald, T.J., Franko, K.L., Brown, J.M. et al. Betamethasone in the Last Week of Pregnancy Causes Fetal Growth Retardation but Not Adult Hypertension in Rats. Reprod. Sci. 10, 469–473 (2003). https://doi.org/10.1016/S1071-55760300151-5
Published:
Issue Date:
DOI: https://doi.org/10.1016/S1071-55760300151-5