Abstract
Objective
Because gonadotropin releasing hormone analogue (GnRHa) therapy often causes leiomyoma regression, in part through alteration of growth factor and receptor expression, we determined whether GnRHa therapy alters the expression of extracellular signal-regulated kinase (ERK) and focal adhesion kinase (FAK), which are linked to intracellular signaling pathways activated by ovarian steroids, growth factors, and adhesion molecules.
Methods
Leiomyoma and matched unaffected myometrium were collected from women who received GnRHa therapy (n = 5) and untreated women (n = 10). We determined the expression of ERK1, ERK2, FAK, phosphorylated ERK (pERK1/2), and pFAK using Western blotting and immunohis-tochemical analysis.
Results
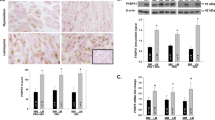
Leiomyoma and myometrium expressed ERK1 (44 kD), ERK2 (42 kD), and FAK (125 kD) at variable levels with increased ERK2, pERK, and FAK expression in leiomyoma. We found that GnRHa therapy resulted in a noticeable decrease in ERK2 and FAK, with significant reduction in pERKl/2 and low or undetectable levels of pFAK in both leiomyoma and myometrium compared with the untreated group (P <.05). Immunohis to chemically ERK1, ERK2, FAK, pERKl/2, and pFAK were localized in smooth muscle cells and connective tissue fibroblasts in GnRHa-treated and untreated leiomyoma and myometrium, with considerable reduction in their intensity as indicated by HScore in GnRHa-treated tissues.
Conclusion
The data provide further evidence that leiomyoma regression induced by GnRHa is mediated in part through a mechanism involving suppression of signal transduction pathways involving growth factors or ovarian steroid and adhesion molecules.
Similar content being viewed by others
References
Kettel LM, Murphy AA, Morales AJ, Rivier J, Vale W, Yen SS. Rapid regression of uterine leiomyomas in response to daily administration of gonadotropin-releasing hormone antagonist. Fertil Steril 1993;60:642–6.
Friedman AJ. Treatment of uterine myomas with GnRH agonists. Semin Reprod Endocrinol 1993;11:154–61.
Takeuchi H, Kobori H, Kikuchi I, Sato Y, Mitsuhashi N. A prospective randomized study comparing endocrinological and clinical effects of two types of GnRH agonists in cases of uterine leiomyomas or endometriosis. J Obstet Gynaecol Res 2000;26: 325–31.
Chegini N. Implication of growth factor and cytokine networks in leiomyomas. In: Hill J, ed. Cytokines in human reproduction. New York: Wiley & Sons, 2000:133–62.
Chegini N, Rong H, Dou Q, Kipersztok S, Williams RS. Gonadotropin releasing hormone (GnRH) and GnRH receptor gene expression in human myometrial and leiomyomta and the direct action of GnRH analogs on myometrial smooth muscle cells interaction with ovarian steroids in vitro. J Clin Endocrinol Metab 1996;81:3215–21.
Raga F, Casan EM, Kruessel JS, et al. Quantitative gonadotropin-releasing hormone gene expression and immunohisto-chemical localization in human endometrium throughout the menstrual cycle. Biol Reprod 1998;59:661–9.
Dou Q, Zhao Y, Tarnuzzer RW, et al. Suppression of TGF-ßs and TGF-ß receptors mRNA and protein expression in leiomy-omata in women receiving gonadotropin releasing hormone agonist therapy. J Clin Endocrinol Metab 1996;81:3222–30.
Upadhyaya NB, Doody MC, Googe PB. Histological changes in leiomyomata treated with leuprolide acetate. Fertil Steril 1990; 54:811–4.
Wu X, Blanck A, Olovsson M, Moller B, Lindblom B. Expression of basic fibroblast growth factor (bFGF), FGF receptor 1 and FGF receptor 2 in uterine leiomyomas and myometrium during the menstrual cycle, after menopause and GnRHa treatment. Acta Obstet Gynecol Scand 2001;80:497–504.
Gustavsson I, Englund K, Faxen M, Sjoblom P, Lindblom B, Blanck A. Tissue differences but limited sex steroid responsiveness of c-fos and c-jun in human fibroids and myometrium. Mol Hum Reprod 2000;6:55–9.
Rintala S, Kujansuu E, Teisala K, Rantala I, Kivinen S, Tuimala R. GnRH analogues and uterine leiomyomas. Effect of hormone replacement therapy on cell proliferation. Gynecol Obstet Invest 1999;48:276–9.
Englund K, Blanck A, Gustavsson I, et al. Sex steroid receptors in human myometrium and fibroids: Changes during the menstrual cycle and gonadotropin-releasing hormone treatment. J Clin Endocrinol Metab 1998;83:4092–6.
Mizutani T, Sugihara A, Nakamuro K, Terada N. Suppression of cell proliferation and induction of apoptosis in uterine leiomyoma by gonadotropin-releasing hormone agonist (leuprolide acetate). J Clin Endocrinol Metab 1998;83:1253–5.
Pearson G, Robinson F, Beers Gibson T, et al. Mitogen-acti-vated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr Rev 2001;22:153–83.
Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem 2001;276: 36869–72.
Lange CA, Richer JK, Horwitz KB. Hypothesis: Progesterone primes breast cancer cells for cross-talk with proliferative or antiproliferative signals. Mol Endocrinol 1999;13:829–36.
Cheng KW, Leung PC. The expression, regulation and signal transduction pathways of the mammalian gonadotropin-releasing hormone receptor. Can J Physiol Pharmacol 2000;78:1029–52.
Kraus S, Naor Z, Seger R. Intracellular signaling pathways mediated by the gonadotropin-releasing hormone (GnRH) receptor. Arch Med Res 2001;32:499–509.
Oh AS, Lorant LA, HollowayJN, Miller DL, Kern FG, El-Ashry D. Hyperactivation of МАРК induces loss of ERa expression in breast cancer cells. Mol Endocrinol 2001;15:1344–59.
Gee JM, Robertson JF, Ellis IO, Nicholson RI. Phosphorylation of ERK1/2 mitogen-activated protein kinase is associated with poor response to anti-hormonal therapy and decreased patient survival in clinical breast cancer. Int J Cancer 2001;95:247–54.
Taylor CV, Letarte M, Lye SJ. The expression of integrins and Cadherins in normal human uterus and uterine leiomyomas. Am J Obstet Gynecol 1996;175:411–9.
Mechtersheimer G, Barth T, Quentmeier A, Moller P. Differential expression of beta 1 integrins in nonneoplastic smooth and striated muscle cells and in tumors derived from these cells. Am J Pathol 1994;144:1172–82.
Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol 1999;71:435–78.
Panetti TS. Tyrosine phosphorylation of paxillin, FAK, and p130CAS: Effects on cell spreading and migration. Frontiers Biosci 2002;7:d143–50.
Schaller MD. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim Biophys Acta 2001; 1540:1–21.
Parsons JT, Martin KH, Slack JK, Taylor JM, Weed SA. Focal adhesion kinase: A regulator of focal adhesion dynamics and cell movement. Oncogene 2000;19:5606–13.
Jones RJ, Brunton VG, Frame MC. Adhesion-linked kinases in cancer; emphasis on src, focal adhesion kinase and PI3-kinase. Eur J Cancer 2000;36:1595–606.
Ruoslahti E. Fibronectin and its integrin receptors in cancer. Adv Cancer Res 1999;76:1–20.
Kornberg LJ. Focal adhesion kinase and its potential involvement in tumor invasion and metastasis. Head Neck 1998;20:745–52.
Marshall DD, Kornberg LJ. Overexpression of scatter factor and its receptor (c-met) in oral squamous cell carcinoma. Laryngoscope 1998;108:1413–7.
Leone M, Cucuccio S, Venturini PL, Valenzano-Menada M, Messeni Leone M, DeCecco L. Immunohistochemical localization of epidermal growth factor receptor in leiomyomas from women treated with goserelin depot. Horm Metab Res 1991;23: 442–5.
Hori M, Inagawa S, Shimazaki J, Itabashi M, Hori M. Overexpression of mitogen-activated protein kinase superfamily proteins unrelated to Ras and AF-1 of estrogen receptor a mutation in advanced stage human breast cancer. Pathol Res Pract 2000; 196: 817–26.
Volmat V, Camps M, Arkinstall S, Pouyssegur J, Lenormand P. The nucleus, a site for signal termination by sequestration and inactivation of p42/p44 MAP kinases. J Cell Sci 2001;114:3433–43.
Albanell J, Codony-Servat J, Rojo F, et al. Activated extracellular signal-regulated kinases: Association with epidermal growth factor receptor/transforming growth factor alpha expression in head and neck squamous carcinoma and inhibition by anti-epidermal growth factor receptor treatments. Cancer Res 2001;61:6500–10.
Gioeli D, Zecevic M, Weber MJ. Immunostaining for activated extracellular signal-regulated kinases in cells and tissues. Methods Enzymol 2001;332:343–53.
Macphee DJ, Lye SJ. Focal adhesion signaling in the rat myometrium is abruptly terminated with the onset of labor. Endocrinology 2000;141:274–83.
Owens LV, Xu L, Marston WA, et al. Overexpression of the focal adhesion kinase (pl25FAK) in the vascular smooth muscle cells of intimai hyperplasia. J Vasc Surg 2001;34:344–9.
Tang DD, Gunst SJ. Selected contribution: Roles of focal adhesion kinase and paxillin in the mechanosensitive regulation of myosin phosphorylation in smooth muscle. J Appl Physiol 2001; 91:1452–9.
Taylor JM, Mack CP, Nolan K, Regan CP, Owens GK, Parsons JT. Selective expression of an endogenous inhibitor of FAK regulates proliferation and migration of vascular smooth muscle cells. Mol Cell Biol 2001;21:1565–72.
Cance WG, Harris JE, Iacocca MV, et al. Immunohistochemical analyses of focal adhesion kinase expression in benign and malignant human breast and colon tissues: Correlation with preinvasive and invasive phenotypes. Clin Cancer Res 2000;6:2417–23.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by a grant (NIH HD37432) from the National Institutes of Health.
Rights and permissions
About this article
Cite this article
Chegini, N., Kornberg, L. Gonadotropin Releasing Hormone Analogue Therapy Alters Signal Transduction Pathways Involving Mitogen-Activated Protein and Focal Adhesion Kinases in Leiomyoma. Reprod. Sci. 10, 21–26 (2003). https://doi.org/10.1016/S1071-5576(02)00184-3
Published:
Issue Date:
DOI: https://doi.org/10.1016/S1071-5576(02)00184-3