Abstract
Background
The androgen receptor (AR) plays essential roles in female reproductive physiology. In vitro studies have shown that AR exerts 2 distinct but related functions in myometrial cells. The AR is required for myometrial cell cycling ligand independently, thereby controls myometrial cell proliferation. The AR also exerts antiapoptotic functions through myeloid cell leukemia 1 (MCL1) in both ligand-dependent and ligand-independent manners. However, these AR functions have not yet been confirmed in vivo. This study provides in vivo evidence that AR expression is associated with myometrial cell proliferation and apoptosis.
Methods
Myometrial tissue was collected on day 6 of pregnancy from mice bearing an intact AR (AR+/+) or mice with AR heterozygous (AR−/+) or homozygous (AR−/−) functional knockout. Human uterine leiomyoma and paired myometrial tissue biopsies (n = 14 patients) were collected during hysterectomy. Immunohistochemistry was used to assess the protein expression of AR, MCL1, and Ki-67 index, as well as the functional associations of AR with MCL1 and Ki-67 index.
Results
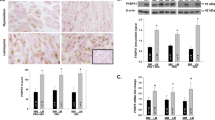
In AR−/− mice, MCL1 protein levels were reduced ∼50% in both circular and longitudinal myometrial cells when compared with that in AR+/+ and AR−/+ mice. By contrast, Ki-67 index remained unchanged. The AR protein expression and Ki-67 index in leiomyoma were ∼2- to 3-fold higher than that in normal myometrium. Although MCL1 expression was not coordinately increased in leiomyoma, strong positive correlations between AR and MCL1 expression were observed in leiomyoma but not in normal myometrium tissue.
Conclusion
These results suggest that AR is an important regulator of myometrial growth in vivo during pregnancy and in leiomyoma.
Similar content being viewed by others
References
Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocrine reviews. 2000;21(5):514–550.
Lye SJ, Tsui P, Dong X, et al.Myometrial programming: a new concept underlying the regulation of myometrial function during pregnancy. In: Petraglia F, Strauss JF III, Gabbe SG, Weiss G, ed. Preterm Birth—Mechanisms, Mediators, Prevention and Interventions. London, UK: Informa UK Ltd; 2007: 1–18.
Shynlova O, Tsui P, Jaffer S, Lye SJ. Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour. Eur J Obstet Gynecol Reprod Biol. 2009;144(suppl 1): S2-S10.
Shynlova O, Oldenhof A, Dorogin A, et al. Myometrial apoptosis: activation of the caspase cascade in the pregnant rat myometrium at midgestation. Biol Reprod. 2006;74(5):839–849.
Islam MS, Protic O, Stortoni P, et al. Complex networks of multiple factors in the pathogenesis of uterine leiomyoma. Fertil Steril. 2013;100(1):178–193.
Bulun SE. Uterine fibroids. N Engl J Med. 2013;369(14): 1344–1355.
Flake GP, Moore AB, Sutton D, et al. The natural history of uterine leiomyomas: light and electron microscopic studies of fibroid phases, interstitial ischemia, inanosis, and reclamation. Obstet Gynecol Int. 2013;2013:528376.
Leppert PC, Jayes FL, Segars JH. The extracellular matrix contributes to mechanotransduction in uterine fibroids. Obstet Gynecol Int. 2014;2014:783289.
Ishikawa H, Ishi K, Serna VA, Kakazu R, Bulun SE, Kurita T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology. 2010;151(6):2433–2442.
Parsanezhad ME, Azmoon M, Alborzi S, et al. A randomized, controlled clinical trial comparing the effects of aromatase inhibitor (letrozole) and gonadotropin-releasing hormone agonist (triptorelin) on uterine leiomyoma volume and hormonal status. Fertil Steril. 2010;93(1):192–198.
Donnez J, Tatarchuk TF, Bouchard P, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366(5):409–420.
Weihua Z, Ekman J, Almkvist A, et al. Involvement of androgen receptor in 17beta-estradiol-induced cell proliferation in rat uterus. Biol Reprod. 2002;67(2):616–623.
Liu L, Li Y, Xie N, et al. Proliferative action of the androgen receptor in human uterine myometrial cells—a key regulator for myometrium phenotype programming. J Clin Endocrinol Metab. 2013;98(1):218–227.
Slomczynska M, Duda M, Burek M, Knapczyk K, Czaplicki D, Koziorowski M. Distribution of androgen receptor in the porcine uterus throughout pregnancy. Reprod Domest Anim. 2008;43(1): 35–41.
Mertens HJ, Heineman MJ, Theunissen PH, de Jong FH, Evers JL. Androgen, estrogen and progesterone receptor expression in the human uterus during the menstrual cycle. Eur J Obstet Gynecol Reprod Biol. 2001;98(1):58–65.
Li H, Li Y, Morin D, Plymate S, Lye S, Dong X. The androgen receptor mediates antiapoptotic function in myometrial cells. Cell Death Dis. 2014;5: e1338.
Walters KA, Allan CM, Jimenez M, et al. Female mice haploin-sufficient for an inactivated androgen receptor (AR) exhibit age-dependent defects that resemble the AR null phenotype of dysfunctional late follicle development, ovulation, and fertility. Endocrinology. 2007;148(8):3674–3684.
Yu Y, Liu L, Xie N, et al. Expression and function of the progesterone receptor in human prostate stroma provide novel insights to cell proliferation control. J Clin Endocrinol Metab. 2013;98(7): 2887–2896.
Yu Y, Yang O, Fazli L, Rennie PS, Gleave ME, Dong X. Progesterone receptor expression during prostate cancer progression suggests a role of this receptor in stromal cell differentiation. Prostate. 2015;75(10):1043–50.
Bae J, Leo CP, Hsu SY, Hsueh AJ. MCL-1 S, a splicing variant of the antiapoptotic BCL-2 family member MCL-1, encodes a proapoptotic protein possessing only the BH3 domain. J Biol Chem. 2000;275(33):25255–25261.
Matsuo H, Maruo T, Samoto T. Increased expression of Bcl-2 protein in human uterine leiomyoma and its up-regulation by progesterone. J Clin Endocrinol Metab. 1997;82(1):293–299.
Burroughs KD, Fuchs-Young R, Davis B, Walker CL. Altered hormonal responsiveness of proliferation and apoptosis during myometrial maturation and the development of uterine leiomyomas in the rat. Biol Reprod. 2000;63(5):1322–1330.
Maruo T, Matsuo H, Samoto T, et al. Effects of progesterone on uterine leiomyoma growth and apoptosis. Steroids. 2000;65(10–11):585–592.
Dixon D, Flake GP, Moore AB, et al. Cell proliferation and apoptosis in human uterine leiomyomas and myometria. Virchows Archiv. 2002;441(1):53–62.
Migliaccio A, Castoria G, Di Domenico M, et al. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO J. 2000;19(20):5406–5417.
Baron S, Manin M, Beaudoin C, et al. Androgen receptor mediates non-genomic activation of phosphatidylinositol 3-OH kinase in androgen-sensitive epithelial cells. J Biol Chem. 2004;279(15): 14579–14586.
Gao Z, Matsuo H, Wang Y, Nakago S, Maruo T. Up-regulation by IGF-I of proliferating cell nuclear antigen and Bcl-2 protein expression in human uterine leiomyoma cells. J Clin Endocrinol Metab. 2001;86(11):5593–5599.
Wu X, Blanck A, Olovsson M, Henriksen R, Lindblom B. Expression of Bcl-2, Bcl-x, Mcl-1, Bax and Bak in human uterine leiomyomas and myometrium during the menstrual cycle and after menopause. J Steroid Biochem Mol Biol. 2002;80(1):77–83.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lan, M., Li, H., Bao, L. et al. In Vivo Evidence of the Androgen Receptor in Association With Myometrial Cell Proliferation and Apoptosis. Reprod. Sci. 23, 264–271 (2016). https://doi.org/10.1177/1933719115602771
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719115602771