Abstract
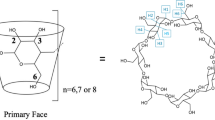
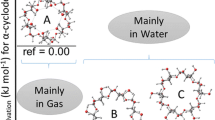
The validity of the “three-point interaction” model is examined in the guest exchange reaction involving complexes of cyclodextrins and amino acids. The amino acid guest is exchanged in the gas phase in the presence of a gaseous alkyl amine. The net reaction is proton transfer between the protonated amino acid and the alkyl amine. The amino acid is lost as a neutral species. This reaction is sensitive to the chirality of the amino acid. Several amino acids are examined as well as the respective methyl esters to determine the role of the three interacting groups (ammonium, carboxylic acid, and side chain) in enantioselectivity. We find that the three-point interaction model is indeed valid in the gas phase. Enantioselectivity is optimal when two points of attraction and one repulsion is present in the gas-phase complex. The results are supported by molecular modeling calculations. A mechanism for the exchange is proposed.
Similar content being viewed by others
References
Hofmeister, G.; Leary, J. A. Org. Mass Spectrom. 1991, 26, 811–812.
Dang, T. T.; Pedersen, S. F.; Leary, J. A. J. Am. Soc. Mass Spectrom. 1994, 5, 452–459.
Reetz, M. T.; Becker, M. H.; Klein, H. W.; Stöckigt, D. Angew. Chem. Int. Ed. 1999, 38, 1758–1761.
Tao, W. A.; Zhang, D.; Wang, F.; Thomas, P. D.; Cooks, R. G. Anal. Chem. 1999, 71, 4427–4429.
Liang, Y.; Bradshaw, J. S.; Izatt, R. M.; Pope, R. M.; Dearden, D. V. Int. J. Mass Spectrom. 1999, 185/186/187, 977–988.
Sawada, M.; Shizuma, M.; Takai, Y.; Yamada, H.; Kaneda, T.; Hanafusa, T. J. Am. Chem. Soc. 1992, 114, 4405–4406.
Sawada, M.; Takai, Y.; Yamada, H.; Kaneda, T.; Kamada, K.; Mizooku, T.; Hirose, K.; Tobe, Y.; Naemura, K. Chem. Commun. 1994, 2497–2498.
Sawada, M. Mass Spectrom. Rev. 1997, 16, 73–90.
Sawada, M.; Takai, Y.; Kaneda, T.; Arakawa, R.; Okamoto, M.; Doe, H.; Matsuo, T.; Naemura, K.; Hirose, K.; Tobe, Y. Chem. Commun. 1996, 1735–1736.
Sawada, M.; Takai, Y.; Yamada, H.; Hirayama, S.; Kaneda, T.; Tanaka, T.; Kamada, K.; Mizooku, T.; Takeuchi, S.; Ueno, K.; Hirose, K.; Tobe, Y.; Naemura, K. J. Amer. Chem. Soc. 1995, 117, 7726–7736.
Fales, H. M.; Wright, G. W. J. Am. Chem. Soc. 1977, 99, 2339–2340.
Denisov, E. N.; Shustryakov, V.; Nikolaev, E. N.; Winkler, F. J.; Medina, R. Int. J. Mass Spectrom. Ion Proc. 1999, 183, 357–368.
Honovich, J. P.; Karachevtsev, G. V.; Nikolaev, E. N. Rapid Commun. Mass Spectrom. 1992, 6, 429–433.
Nikolaev, E. N.; Goginashvily, G. T.; Talrose, V. L.; Kostjanovsky, R. G. Int. J. Mass Spectrom. Ion Processes 1988, 86, 249–252.
Nikolaev, E. N.; McMahon, T. B. 43rd ASMS Conference on Mass Spectrometry and Allied Topics; Atlanta, GA, May 21–26, 1995.
Chu, I. H.; Dearden, D. V.; Bradshaw, J. S.; Huszthy, P.; Izatt, R. M. J. Am. Chem. Soc. 1993, 115, 4318–4320.
Dearden, D. V.; Dejsupa, C.; Liang, Y. J.; Bradshaw, J. S.; Izatt, R. M. J. Am. Chem. Soc. 1997, 119, 353–359.
Camara, E.; Green, M. K.; Penn, S. G.; Lebrilla, C. B. J. Am. Chem. Soc. 1996, 118, 8751–8752.
Gong, S.; Camara, E.; He, F.; Green, M. K.; Lebrilla, C. B. Int. J. Mass Spectrom. Ion Processes 1999, 185/186/187, 401–412.
Ramirez, J.; He, F.; Lebrilla, C. B. J. Am. Chem. Soc. 1998, 120, 7387–7388.
Dalgliesh, C. E. J. Chem. Soc. 1953, 3940–3952.
Kitae, T.; Nakayama, T.; Kano, K. J. Chem. Soc., Perkin Trans. 2 1998, 207–212.
Li, S.; Purdy, W. C. Chem. Rev. 1992, 92, 1457–1490.
Kano, K.; Kitae, T.; Takashima, H. Inclusion Phenom. Mol. Recognit. Chem. 1996, 25, 243–248.
Ramirez, J.; Ahn, S.; Grigorean, G.; Lebrilla, C. B. J. Am. Chem. Soc., 2000, 122, 6884–6890.
Easson, E. H.; Stedman, E. Biochemistry 1933, 27, 1257.
Ogston, A. G. Nature 1948, 4129, 963.
Sokolov, V. I.; Zefirov, N. S. Dokl. Akad. Nauk 1991, 319, 1382–1383.
Booth, T. D.; Wahnon, D.; Wainer, I. Chirality 1997, 9, 96–98.
Davankov, V. Chirality 1997, 9, 99–102.
Ciucanu, I.; Kerek, F. Carbohydr. Res. 1984, 1984, 209–217.
Hoogwater, D. A.; Peereboom, M. Tetrahedron 1990, 46, 5325–5332.
Gard, E. E.; Green, M. K.; Warren, H.; Camara, E. J. O.; He, F.; Penn, S. G.; Lebrilla, C. B. Int. J. Mass Spectrom. Ion Processes 1996, 158, 115–127.
Carroll, J. A.; Penn, S. G.; Fannin, S. T.; Wu, J.; Cancilla, M. T.; Green, M. K.; Lebrilla, C. B. Anal. Chem. 1996, 68, 1798–1804.
Hunter, E. P. L.; Lias, S. G. J. Phys. Chem. Ref. Data 1998, 27, 413–656.
Still, W. C.; Kilburn, J. D.; Sanderson, P. E. J.; Liu, R.; Wiley, M. R.; Hollinger, F. P.; Hawley, R. C.; Nakamima, M.; Bernardi, A.; Hong, J. I.; Namgoong, S. K. Isr. J. Chem. 1992, 32, 41–45.
Zhang, X. X.; Bradshaw, J. S.; Izatt, R. M. Chem. Rev. 1997, 97, 3313–3361.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahn, S., Ramirez, J., Grigorean, G. et al. Chiral recognition in gas-phase cyclodextrin: Amino acid complexes—Is the three point interaction still valid in the gas phase?. J Am Soc Mass Spectrom 12, 278–287 (2001). https://doi.org/10.1016/S1044-0305(00)00220-8
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/S1044-0305(00)00220-8