Abstract
Root plasticity enables plants to adapt to spatial and temporal changes in soil resources. In this study, 40 common bean genotypes evaluated for two root and shoot traits under irrigated and water stress. Three genotypes WB-216, WB-N-2, and WB-966 with contrasting plasticity responses were used for in-depth study. Highest positive plasticity for most root traits was found in case of WB-N2 and WB-216, whereas, WB-966 had negative plasticity for all the traits recorded. In terms of spatial plasticity for root traits in three root length sections, WB-216 was positively plastic for root diameter with progressive decrease from top to bottom sections. WB-N2 had positive plasticity values for root diameter, root surface area and root volume. WB-966 had negative plasticity for all the traits. For WB-216, the root diameter increased under drought in S1 but was almost same in bottom sections. In case of WB-N2, there was increase in root diameter in S2 and S3, but for WB-966, root diameter decreased in all sections. Similar trend was observed in all three genotypes for root surface area and volume. We report that major drivers of spatial plasticity of root architectural traits are increased root diameter, surface area and volume at deeper layers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Common bean (Phaseolus vulgaris L.), is an important component of human food, nutrition, health and livelihood systems. With around 35.92 million hectares from 27.71 million tonnes, with an average productivity of 7716 kg ha−1, it is one of the most important legume crops of the world, with Asia leading the production, followed by North, Central, and South America and Africa [1]. However, water stress affects global common bean production by nearly 60% [2], with complete crop failure under severe stress conditions [3]. With common bean being invariably grown in rainfed ecosystems, where water stress is the predominant factor affecting crop yield [4], yield decreases are driven by the duration and severity of drought stress. Water stress implicates bean yield directly by tissue dehydration and indirectly by reducing nutrient absorption [5]. The reduction in yield is associated with decrease in the number of pods per plant, number of seeds per pod and 100-seed weight [6, 7]. Since common bean is grown invariably under low input farming system, and the crop is not known for its inherent drought tolerance, it is imperative to develop drought resilient genotypes for sustainable bean production. This warrants in depth studies on understanding the mechanism of drought tolerance in beans.
Root system architecture (RSA) represents the spatial structure of the root system or the explicit placement of root axes [8], and an optimized RSA is crucial for plant growth and agricultural productivity [9]. RSA not only provides for the uptake of water and nutrients but also acts as the first line of defense against many abiotic stresses [10, 11]. As a general observation, deep roots have been reported to be the most consensual trait that contributes to drought avoidance in stress conditions through effective foraging of mineral resources and water from deeper layers and helps the plant to respond to the evaporative demand [12]. In fact, the development of stress-resilient crops is driven by effective RSA, composed of structural characteristics such as root length, spread, branching, growth angle, and the quantity and length of lateral roots [13]. There is also a need to understand the effect of better root architecture on the efficiency of physiological and biochemical processes that help plants to reduce the risk of failure of key growth, development and reproductive fitness processes, and this will help breeders to develop an integrated selection index to use surrogate traits for selection of stress resilient bean genotypes [13]. RSA has important roles in the soil resources acquisition [14]. However, there are important trade-offs between various resources such as water and nitrogen (mobile) and phosphorus (immobile). As an example, steep root angles may have an adaptive advantage for capturing deeper resources such as water and nitrogen, but may be maladaptive for phosphorus capture, where shallow growth angles can improve topsoil foraging and capture of phosphorus [15]. In common bean, genotypes with higher basal root number and shallower growth angle improve phosphorus acquisition, but, increased number of basal roots imposes carbon limitation leading to a reduced root depth and therefore implicating capture of deep resources such as water and nitrogen [16].
More than the root phenes per se, root phenotypic plasticity is an important phenomenon for the optimized capture of edaphic resources, an enables plants to adapt to spatial and temporal changes in resources in their immediate environments. Most of the studies on phenotypic plasticity are based on measurement of allometric traits such as length, biomass and volume [17]. However, the plastic response may not always be adaptive and can sometimes be counterproductive (maladaptive plasticity). Plasticity in root traits can potentially influence the fitness of a genotype in response to water stress [18]. The phenotypic plasticity may include components of genotype by environment interaction, adaptation, and acclimation.
Plasticity response may be adaptive (positively associated with fitness), maladaptive (counterproductive), or neutral (no apparent advantage) in regard to fitness to resource constraints. In the soil system, many root traits and their combinations may confer adaptive advantage for resource capture through plastic responses, that enables a genotype to produce better adapted phenotypes and phenotype-environment combinations. Genotypes either exhibit perfect plasticity by expressing the more adaptive traits or combinations of traits in various environments without any cost (positively associated with fitness). However, in many instances, plasticity can be maladaptive or neutral [19] and have additional costs for increased fitness value without any apparent adaptive value and we would only expect costly forms of plasticity to persist if they have fitness value. Maladaptive plasticity refers to a plastic response that is adaptive in an evolutionary context, but is counterproductive in a new environment. As an example, if plants change root system under drought stress by an allomeric shift towards deeper layers, it may lead to compromised P capture in top soil and may be counterproductive. That is probably the reason that breeders invariably look for a parsimonious root system [20].
Selection for root phene plasticity could be a viable strategy for breeding programs, if selection is done in specific targeted environments or under specific stresses environments. However, selection of genotypes that exhibit adaptive plasticity under water stressed environments may or may not have same adaptive capacity such as nutrient stress. Moreover, the screening for plasticity should be done for traits or a combination of traits that improve final economic trait viz., yield or other indicators of plant performance. Breeding for plasticity may be maladaptive in environments with multiple stresses or stresses that fluctuate on short time scales or that vary throughout the growth season. In the present study we used three genotypes with contrasting root architectural plasticity to understand the causative root phenes and the effect on overall genotypic plasticity under water stress.
2 Methods
2.1 Materials
Two independent experiments were conducted. In the first experiment, forty diverse genotypes of common bean were used. In the second experiment, three contrasting genotypes of common bean, namely WB-216, WB-N-2, and WB-966 were used understand the influence of water stress on root plasticity. WB-216 (SKU-RB-39) and WB-N-2 (PAS-923) are the local landraces preserved in germplasm bank, and WB-966 (G-1295) is a germplasm accession procured from CIAT (International Center for Tropical Agriculture), Columbia. WB-216 is a bold seeded pink kidney shaped genotypes with stay green trait. WB-N2 is a small seeded brilliant red cuboidal genotype with deep rooting trait. WB-966 is a mottled cranberry type kidney shaped genotype with resistance to anthracnose disease.
S. No. | Genotype | Type of material | Pedigree | Origin | Gene pool |
|---|---|---|---|---|---|
1 | WB-216 | Local landrace | SKU-RB-39 | SKUAST-Kashmir | Andean |
2 | WB-N2 | Local landrace | PAS-20-112 | SKUAST-Kashmir | Mesoamerican |
3 | WB-966 | Exotic germplasm | G-1295 | CIAT Columbia | Andean |
2.2 Growth conditions
Root and physiological traits and reproductive success: The experiment was conducted at the Greenhouse facility of SKUAST-K with three replications for each treatment. Four seeds were grown in 1.5 m tall PVC (polyvinyl chloride) columns with an internal diameter of 20 cm. The columns were filled with equal portions of local field soil and sand, allowing for easy root separation and a creating near-equal replica of field conditions. In each column, 10 g of a slow release fertilizer Osmocote (19:6:12 N:P2O5:K2O) was evenly mixed at the top 2 cm soil portion before sowing. In order to manage sucking insects, one gram of a systemic insecticide, Marathon 1% G (a.i.: Imidacloprid), was applied. The surface sterilized seeds were sown in each column at 4 cm depth and irrigated continuously until the crop reached the first trifoliate leaf stage. At this stage, one competitive plant was retained per column.
Imposition of water stress: Non-stress treatment (well-watered) plants were maintained at 80% FC by irrigating water daily from sowing to harvest. In case of water stress treatment, the plants were maintained under 80% FC up to the trifoliate stage. Stress was imposed by stopping water till the final harvest of the experiment (48 days after sowing; DAS). The duration of the water stress was 41 days. The moisture content of the columns, quantified on a weight basis [21], at the end of the water stress treatment was 30%.
Root architectural studies: At the end of the experiment (48 days), the roots from each column were harvested by gently tilting the columns at about 140 ℃ as per the methodology of [22] to ensure that the growing medium along with roots can slip out of the column, so that the intact roots can be separated from the growing medium. The upper part of the plant was separated by cutting at the base of the stem to harvest the roots and allow relative measurement of above and below ground plant parts. The roots were spread to measure their length from the base of the stem to the longest tip of the root system to estimate the rooting depth. The roots were washed with water to remove soil/sand, and sealed in Ziploc bags, and stored at 4 ℃. For detailed root analysis using scanning, each root was sliced into three equal length sections (S1, S2 and S3) and each section was submerged in a water and carefully spread to ensure maximum root separation and minimize overlap and scanned with an Epson photo scanner (Epson Perfection V700, with resolution of 6400 dpi). The root images were analysed using Rhizo-Vision Explorer Version 2.03 [23] to estimate root traits. The images were acquired on grayscale to a resolution of 700 dpi and analysis was done by setting higher level of rough edge and noise removal.
Evaluation for yield traits: The genotypes were grown in 2023 kharif seasons between May to September at Faculty of Agriculture Research farm (34° 17′ N and 74° 33′ E at an altitude of 1594 msl). The soil of the site is an inception with a clayey-loam texture. The pH of site was normal (7.2) with organic carbon (0.65%), electrical conductivity (0.18 dS/m) and CEC (16 meq/kg). All the genotypes were grown as four-row plots of 4 m length, with 20 cm × 40 cm spacing. The minimum and maximum temperatures varied from 9.00 to 15.01 °C and 21.6 to 34.50 °C respectively. The total precipitation during the crop period was 397.40 mm respectively. The mean minimum (RH1) and maximum (RH2) relative humidity recorded were 55.25% and 79.36% respectively. The non-stressed treatments being regularly irrigated till maturity whenever soil moisture depleted below 30% field capacity, whereas, in the case of water stress treatments, water was withheld 5 days before anthesis till pod formation and after that stopped until maturity. Hand weeding was done to control weeds, and the recommended fertilizer dose was applied as per the university package of practices for common bean.
Experimental design: The experiment in the greenhouse conditions was conducted in a CRD factorial design with genotypes and water regime as two factors, with each treatment replicated three times. In the fiend experiment, the genotypes were evaluated in paired comparison with the non-stressed treatments.
Measurements monitored: In the greenhouse experiment root traits monitored were rooting depth, root biomass, total root length, average root diameter, root volume, and surface area (SA). In case of field experiment data was recorded on ten competitive plants from each genotype for pods plant-1, pod length, seeds pod−1, 100-seed weight and seed yield plant−1 as per the standard procedures [24].
The phenotypic plasticity of all traits was calculated as a relative change in water- stress compared with control conditions using the following formula [25]:
2.3 Data analyses
The analyses of data were performed using the R software (v4.1.2; R Core Team, 2022) and JASP (Ver. 0.17.2, CIMMYT). Results on leaf temperature and relative water content recorded on 14, 21, and 28 days after the imposition of water stress, had similar responses and significance levels for both traits. The Levene’s test was used to check the homogeneity of variances and Shapiro–Wilk’s test was used to check the normality of the data. The root traits, physiological parameters and yield traits were analyzed using a general analysis of variance. The standard error was used as an estimate of variability, and the means of recorded parameters were separated for significance by an LSD test (p < 0.05).
3 Results
3.1 Genetic variability for root plasticity
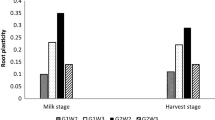
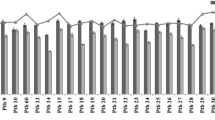
The data pertaining to plasticity on root and shoot traits is presented in Table 1 and Fig. 1. For rooting depth, the mean plasticity was − 0.006 with values ranging from − 0.514 to 0.615. The highest positive plasticity value for root depth was recorded for WB-N2 (0.615), followed by WB-6 (0.602) and WB-54 (0.458) and the lowest value was recorded in WB-413 (− 0.514). For root biomass, the mean plasticity was − 0.215 with values ranging from − 0.757 to 0.301. Highest positive plasticity value was recorded for WB-216 (0.301), followed by WB-956 (0.217) and WB-6 (0.214) and the lowest value was recorded in WB-966 (− 0.757). For plant height, the mean plasticity was − 0.215 with values ranging from − 0.723 to 0.104. Only WB-N2 (0.104) had positive plasticity value. The lowest value was recorded in WB-1310 (− 0.723). Similarly, for shoot biomass, the mean plasticity was − 0.215 with values ranging from − 0.775 to 0.216, with positive plasticity value recorded only for WB-N2 (0.216), whereas the lowest value was recorded in WB-164 (− 0.775). The mean plasticity index based on average of the four traits recorded ranged from − 0.536 to 0.172, with a mean value of − 0.291. Only two genotypes WB-N2 (0.172) and WB-6 (0.025) had positively plasticity index values. Lowest value was recorded for WB-966 (− 0.529).
3.2 Mechanism of drought tolerance based on plasticity in root, shoot and yield traits
The rooting depth of WB-216 does not vary significantly between irrigated control and drought stress (71 vs. 83 cm). However, WB-N2 had a significantly deeper root system under drought (84 cm) than irrigated control (52 cm). The genotype WB-966 had a significantly shallower root system under drought (41 cm) than under drought stress conditions (67 cm) (Fig. 2). The estimates of plasticity for root shoot and yield traits are presented in Table 2. Among root and shoot traits, WB-N2 had positive values of plasticity for plant height (0.62) followed by root biomass (0.22) and root depth (0.10), while WB-216 was positively plastic for shoot biomass (0.30) and plant height (0.17). WB-966 had a negative value of plasticity for all the traits recorded, with the highest negative values recorded for shoot biomass (− 0.76), followed by root biomass (− 0.51), root depth (− 0.46) and plant height (− 0.39). For yield traits, all the genotypes had negative values of plasticity, with the highest negative value recorded for seed yield per plant in case of WB-966 (− 0.33), followed by seeds per pod (− 0.24) and pod length (− 0.22). The overall mean plasticity for WB-216, WB-N2 and WB-966 was − 0.095, − 0.015 and − 0.389 respectively.
3.3 Pattern of variation for plasticity in root trait variation in root traits in different root depth sections
The pattern of spatial plasticity for root traits scored in three root depth sections (S1, S2 and S3) in common bean genotypes with contrasting plasticity responses is given in Table 3. The genotype WB-216 that had comparable root depths in irrigated and water stressed conditions was positively plastic for root diameter with progressive decrease from top to bottom sections. Root surface area decreased in S3 and root volume and root length decreased in S1 and S2. For the genotype WB-N2 that had deeper roots under drought had positive plasticity values for root diameter, root surface area and root volume in S2 sections, even though it was negatively plastic for these traits in S1 section. However, there was a progressive increase in negative values of plasticity for root length. For WB-966 with shallower roots under water stress, all the traits had negative plasticity values in all root sections with higher negative values recorded in S1 section.
The pattern of variation for root traits in three contrasting bean genotypes is presented in Figs. 3 and 4. For WB-216, the root diameter increased under drought in S1 but was almost same in bottom sections. In case of WB-N2, there was increase in root diameter in S2 and S3, while as, in case of WB-966, there was decrease in root diameter in all sections with larger decrease in S1 region. Similar trend was observed in all three genotypes for root surface area. In case of root volume, WB-216 had higher root volume under drought in S1 region, with almost comparable values in in S2 and S3 region. In WB-N2, the root volume increased in S2 and S3, while as in WB-966, the root volume was lower in all sections, with significant decreases in S2 and S3. For total root length, all three genotypes had comparable values in S1. However, the responses were different in sections S2 and S3, with comparable values recorded in WB-216, and decreased root length under water stress, with larger decreases recorded in case of WB-966 in S3.
4 Discussion
In the present study we attempted to elucidate possible role of spatial variability of root traits in determining differential yield responses in common bean genotypes under drought stress. Based on the hypothesis that plasticity of roots is important in conferring plants advantage of resource capture under stress and consequently improving reproductive fitness, we studied the plasticity of root traits in three contrasting genotypes WB-216, WB-N2 and WB-966 in relation to the overall plasticity of yield traits and plasticity of spatial distribution of root traits. We found that spatial plasticity of root traits significantly influences the yield parameters under drought stress and that major drivers of spatial plasticity of root architectural traits are increased root diameter, surface area and volume at deeper layers. Under drought stress, the major functional role of root plasticity is to help plants capture the available water efficiently during progressive soil drying to maintain leaf water status. This is possible, as long as such root responses due to plasticity matched the soil moisture available in the soil profile, which is dependent on the depth of soil and topography [26, 27].
Root traits have important roles in soil resource capture, especially in environments with suboptimal water and nutrient availability and determine the temporal and spatial distribution of root foraging ability in various soil strata to capture mobile and immobile resources [14, 28, 29]. Water and nitrate, being mobile, are invariably available more in deeper soil strata over time on account of evaporation and transpiration losses as well as leaching. However, phosphorus (immobile) and potassium (partially mobile) are more predominant in the topsoil [30]. Plants that produce comparable or higher yields under stress, are able to do so, mainly through acquisition of soil resources at reduced metabolic costs, by optimising the allometric shifts of resource allocation for growth, resource acquisition, and reproductive fitness [14]. With regard to functional role of root traits for resource capture, it is well evidenced that deeper root growth angle drives greater root depth and consequent improved performance under water and nitrogen constraints [31,32,33], whereas, shallower root growth angles are more advantageous for foraging topsoil P and K [15, 30]. Deeper rooting helps in greater spatial root distribution at deeper soil layers and also promotes water uptake through hydraulic lift that drives up water in the day to be used by plants at night [34], with an additional benefit on nitrogen uptake [35].
The root phene diversity and its role in differential soil exploration can serve as an important selection parameter for developing novel root phenotypes that confer improved soil resource capture [36]. However, a measure of phenotypic plasticity as an indicator of response plasticity of allometric traits (length, volume, or biomass), not translated into adaptive value may not have any breeding value. Breeders also need to study any trade-offs or constraints that may limit the breeding value of plasticity response, that accrues from adaptive traits or trait combinations, without additional metabolic cost. Further studies may be needed to understand biochemical and molecular basis of such plasticity and how it mediates improved reproductive fitness and better yields under drought stress. In our earlier studies, we have demonstrated strong influence of deeper roots on biochemical pathways as well as improved above ground traits and physiological attributes such as canopy temperature depression, leaf water status and membrane stability [37].
5 Conclusion
We report that major drivers of spatial plasticity of root architectural traits are increased root diameter, surface area and volume at deeper layers. Greater root diameter of tap and basal roots helps in penetration to deeper layers, whereas, greater surface area and volume help plants increase the area explored through a give root mass and improve water capture in deeper layers, especially under water stress conditions, where water is depleted at a fast pace due to transpiration demand and surface evaporation.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
FAO. FAOSTAT. 2022. www.fao.org/faostat//en/#data/QCL
Urrea CA, Yonts CD, Lyon DJ, Koehler AE. Selection for drought tolerance in dry bean derived from the Mesoamerican gene pool in western Nebraska. Crop Sci. 2009;49:2005–10.
Broughton WJ, Hernandez G, Blair MW, Beebe SE, Gepts P, Vanderleyden J. Beans (Phaseolus spp.)—model food legumes. Plant Soil. 2003;252:55–128.
NIDIS. Current conditions-global water stress information system. 2020. https://www.waterstress.gov/gdm/currentconditions. Accessed 4 June 2020.
Hose E, Clarkson DT, Steudle E, Schreiber L, Hartung W. The exodermis: a variable apoplastic barrier. J Exp Bot. 2001;52(365):2245–64.
Asfaw A, Blair MW. Quantification of drought tolerance in Ethiopian common bean varieties. Agric Sci. 2014;5:124–39.
Khaghani S, Bihamata MR, Rahim F, Dorry HR. Study of qualitative and quantitative traits in red bean in non-stress and drought condition. Asian J Plant Sci. 2008;7:563–8.
Lynch J. Root architecture and plant productivity. Plant Physiol. 1995;109:7–13.
Wang L, Zhu J, Li X, Wang S, Wu J. Salt and water stress and ABA responses related to bZIP genes from V. radiata and V. angularis. Gene. 2018;651:152–60.
Bisht N, Tiwari S, Singh PC, Niranjan A, Chauhan PS. A multifaceted rhizobacterium Paenibacillus lentimorbus alleviates nutrient deficiency-induced stress in Cicer arietinum L. Microbiol Res. 2019;223:110–9.
Daryani P, Darzi Ramandi H, Dezhsetan S, Mirdar Mansuri R, Hosseini Salekdeh G, Shobbar ZS. Pinpointing genomic regions associated with root system architecture in rice through an integrative meta-analysis approach. Theor Appl Genet. 2022. https://doi.org/10.1007/s00122-021-03953-5.
Price AH, Cairns JE, Horton P, Jones HG, Griffiths H. Linking drought-resistance mechanisms to drought avoidance in upland rice using a QTL approach: progress and new opportunities to integrate stomatal and mesophyll responses. J Exp Bot. 2002;53(371):989–1004.
Sofi PA, Mir RA, Bhat KA, Mir RR, Fatima S, Rani S, Zargar SM. From domestication syndrome to breeding objective: insights into unwanted breakup in common beans to improve shattering. Crop Pasture Sci. 2022. https://doi.org/10.1071/CP22130.
Lynch JP. Root phenotypes for improved nutrient capture: an underexploited opportunity for global agriculture. New Phytol. 2019;223(2):548–64.
Ho MD, Rosas JC, Brown KM, Lynch JP. Root architectural tradeoffs for water and phosphorus acquisition. Funct Plant Biol. 2005;32:737–48.
Rangarajan H, Postma JA, Lynch JP. Co-optimization of axial root phenotypes for nitrogen and phosphorus acquisition in common bean. Ann Bot. 2018;122:485–99.
Schneider HM, Klein SP, Hanlon MT, Kaeppler S, Brown KM, Lynch JP. Genetic control of root anatomical plasticity in maize. Plant Genome. 2020;13(1): e20003.
Weiner J. Allocation, plasticity and allometry in plants. Perspect Plant Ecol Evol Syst. 2004;6:207–15.
DeWitt TJ, Sih A, Wilson DS. Costs and limits of phenotypic plasticity. Trends Ecol Evol. 1998;13:77–81.
Schneider HM, Lynch JP. Should root plasticity be a crop breeding target? Front Plant Sci. 2020;15(11):546. https://doi.org/10.3389/fpls.2020.00546.
Black CA, editor. Method of soil analysis, part 2, chemical and microbiological properties. Madison: American Society of Agronomy Inc; 1965.
Sofi PA, Djanaguiraman M, Siddique KHM, Prasad PVV. Reproductive fitness in common bean (Phaseolus vulgaris L.) under drought stress is associated with root length and volume. Ind J Plant Physiol. 2018;23:796–809.
Seethepalli A, Dhakal K, Griffiths M, Guo H, Freschet GT, York LM. RhizoVision explorer: open-source software for root image analysis and measurement standardization. AoB Plants. 2021;13(6): plab056.
Sofi PA. Evaluation standards of Rajmash. Srinagar: SKUAST-K & IIPR publication; 2019. p. 44.
Sandhu N, Raman KA, Torres RO, Audebert A, Dardou A, Kumar A, Henry A. Rice root architectural plasticity traits and genetic regions for adaptability to variable cultivation and stress conditions. Plant Physiol. 2016;171(4):2562–76.
Kameoka E, Suralta RR, Mitsuya S, Yamauchi A. Developmental plasticity of rice root system grown under mild drought stress condition with shallow soil depth; comparison between nodal and lateral roots. Plant Prod Sci. 2016;19(3):411–9.
Menge DM, Kameoka E, Kano-Nakata M, Yamauchi A, Asanuma S, Asai H, Makihara D. Drought-induced root plasticity of two upland NERICA varieties under conditions with contrasting soil depth characteristics. Plant Prod Sci. 2016;19(3):389–400.
Lynch JP, Brown KM. New roots for agriculture: exploiting the root phenome. Phil Trans R Soc B Biol Sci. 2012;367(1595):1598–604.
Lynch JP, Wojciechowski T. Opportunities and challenges in the subsoil: pathways to deeper rooted crops. J Exp Bot. 2015;66:2199–210.
Lynch JP, Brown KM. Topsoil foraging: an architectural adaptation of plants to low phosphorus availability. Plant Soil. 2001;237:225–37.
York LM, Nord EA, Lynch JP. Integration of root phenes for soil resource acquisition. Front Plant Sci. 2013;4:355.
Trachsel S, Kaeppler SM, Brown KM, Lynch JP. Maize root growth angles become steeper under low N conditions. Field Crop Res. 2013;140:18–31.
Dathe A, Postma JA, Postma-Blaauw MB, Lynch JP. Impact of axial root growth angles on nitrogen acquisition in maize depends on environmental conditions. Ann Bot. 2016;118(3):401–14.
Caldwell MM, Dawson TE, Richards JH. Hydraulic lift: consequences of water efflux from the roots of plants. Oecologia. 1998;113:151–61.
White JC, Liste HH. Plant hydraulic lift of soil water–implications for crop production and land restoration. Plant Soil. 2008;313:1–17.
Jochua CN, Strock CF, Lynch JP. Root phenotypic diversity in common bean reveals contrasting strategies for soil resource acquisition among gene pools and races. Crop Sci. 2020;60(6):3261–77.
Shafi S, Zaffar A, Riyaz I, Shikari AB, Najeeb S, Zargar SM, Djanaguiraman M, Gurumurthy S, Prasad PVV, Sofi PA. Differential drought responses in deep and shallow-rooted rice genotypes: enzymatic and non-enzymatic insights. Plant Physiol Rep. 2024. https://doi.org/10.1007/s40502-024-00788-2.
Author information
Authors and Affiliations
Contributions
PAS: Conceived the research and design of experiment, IR, SS, AZ: Executed the experiment, PAS, MAW, SMZ, MD: Analysed the data and lead the manuscript preparation, PAS, SMZ, MD, PVVP: Helped in the manuscript preparation.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The germplasm used in the present study were procured from National Gene Bank of India (NBPGR) as well as international gene banks (CIAT, Columbia) and Landraces collected from various potential areas of bean variability in Kashmir valley. The landrace samples are collected during exploration programmes duly permitted and approved by the university and samples are documented properly. SKUAST-Kashmir has been established by an act of legislature and is the custodian of crop biodiversity. As a member of the University and head of Plant Breeding Department, Corresponding author (PAS) is fully authorised to collect, conserve and characterise the local crop genetic resources for breeding improved varieties. All the guidelines were followed as per the University research ethics for collection, characterisation and documentation of landraces or germplasm accessions.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Riyaz, I., Shafi, S., Zaffar, A. et al. Differential spatial plasticity response in common bean (Phaseolus vulgaris L.) root architecture under water stress is driven by increased root diameter, surface area and volume at deeper layers. Discov. Plants 1, 6 (2024). https://doi.org/10.1007/s44372-024-00006-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44372-024-00006-1