Abstract
Regulators approve newer medications and medication combinations for the treatment of various disorders. For the treatment of anal fissures, a novel combination of currently available medications, such as lidocaine HCl and diltiazem HCl, has been approved. The current work represents high-performance thin layer chromatographic method to estimate the amounts of lidocaine hydrochloride and diltiazem hydrochloride in a combined gel dosage form called Crema-L gel (2% W/W of lidocaine and 2% W/W of diltiazem hydrochloride). This method has been validated. The mobile phase in this method consisted of a mixture of toluene, methanol, ethyl acetate (7:2: 1% v/v/v) and two drops of ammonia. The stationary phase was pre-coated aluminium sheet with silica gel G60 F254 (10 × 10 cm, 0.2 mm layer thickness). The drugs’ resolved peaks were found at the Rf values of 0.59 for Lidocaine HCl and 0.48 for Diltiazem HCl. The overlay UV spectra of the two medications were used to determine the analytical wavelength and at 220 nm, both drugs showed appreciable absorbance. The concentration range of 400–1200 ng/band was determined to be the linear approach for both lidocaine HCl and diltiazem HCl, as indicated by their respective correlation coefficients (r2) of 0.9987 and 0.9980. The technique for simultaneously estimating lidocaine hydrochloride and diltiazem hydrochloride in bulk and pharmaceutical dosage form was shown to be robust, accurate, precise, and repeatable.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
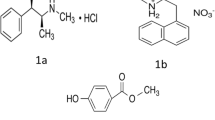
Lidocaine HCl (LID) and Diltiazem HCl (DIL) are two distinct pharmaceutical compounds with different chemical compositions and therapeutic actions, yet both are crucial in medical treatment of anal fissure. LID, chemically identified as 2-(diethylamino)-N-(2,6-dimethylphenyl) acetamide hydrochloride with the empirical formula C14H23ClN2O, functions primarily as a local anaesthetic and cardiac depressant used to manage arrhythmias [1]. On the other hand, DIL, chemically [(2S,3S)]-5-[3-(dimethylamino)ethyl]-2-(4-methoxyphenyl)-4-oxo-2,3-dihydro-1,5-benzothiazepine-3-yl]acetate hydrochloride with the empirical formula C22H27ClN2O4S, acts as a calcium-channel blocker and vasodilator, relaxing smooth muscles in blood vessels [2]. Structures of LID and DIL have been shown in Fig. 1a and b, respectively.
The combination of LID and DIL in rectal formulations is particularly beneficial for treating anal fissures [3], offering both therapeutic relief and symptomatic improvement. LID provides numbing effects to alleviate pain, while DIL works to relax smooth muscles, aiding in healing and reducing discomfort.
Analytical techniques for estimating LID and DIL individually have been extensively documented in the literature [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21], including methods such as ultraviolet spectroscopy, high performance liquid chromatography (HPLC), reversed-phase HPLC (RP-HPLC), HPLC–MS/MS, and others. LID and DIL, both have been estimated in combination with other drugs by UV, HPLC and HPTLC, but only one UV method has been published for estimation of both simultaneously [22]. However, simultaneous estimation methods for both drugs by chromatographic means were lacking until recently. The challenge in developing a simultaneous estimation method lies in the similar physicochemical properties of LID and DIL, particularly their comparable log P (partition coefficient) and pKa values. These similarities make it difficult to achieve adequate separation using chromatographic techniques. Despite this challenge, their structural differences have proven beneficial in achieving resolution between their peaks. A recent study addressed this gap by developing and validating a high performance thin layer chromatographic (HPTLC) method for the simultaneous estimation of LID and DIL, adhering to guidelines outlined in ICH Q2 (R1) [23]. This method not only provides a means to quantify both drugs in a single analysis but also ensures accuracy and reliability in their measurement. The availability of such a method is crucial for pharmaceutical research and clinical practice, enabling efficient formulation development, quality control, and pharmacokinetic studies involving LID and DIL in combination therapies. It represents a significant advancement in analytical chemistry applied to pharmaceuticals, offering a practical solution to the challenges posed by the simultaneous estimation of structurally similar yet functionally distinct compounds like LID and DIL.
2 Experimental
2.1 Standard API, chemicals and materials
The active pharmaceutical ingredients (APIs) of LID and DIL were obtained as complimentary samples from reputable pharmaceutical companies based in Gujarat, India. The marketed gel formulation, known as Crema-L gel, containing 2% w/w of Lidocaine HCl and 2% w/w of Diltiazem HCl, was purchased from a local pharmacy. Chemicals including acetonitrile, toluene, methanol, ethyl acetate, and ammonia were procured from SRL Chemicals Ltd., located in Mumbai, Gujarat.
2.2 Analytical wavelength selection
LID and DIL dissolved readily in acetonitrile solvent. Individual solutions of LID and DIL were prepared at concentrations of 10 µg/ml for analytical purposes. Using a UV–Visible double beam spectrometer (UV-1900i), both solutions were scanned across a wavelength range from 400 to 200 nm. An iso-absorptive point was identified at 220 nm, leading to its selection as the wavelength for quantifying both LID and DIL.
2.3 HPTLC system
The HPTLC setup included a semi-automatic instrument featuring a Linomat 5 sample applicator (CAMAG, Switzerland), a CAMAG TLC scanner IV, and a 100 µl applicator syringe from Hamilton (Switzerland). Chromatographic development was performed using HPTLC aluminium plates sized at 10 cm × 10 cm, pre-coated with silica gel G60-F254 (E. Merck, Darmstadt, Germany; supplied by Anchrom Technologists, Mumbai, India), and twin-through chambers.
2.4 Standard solution preparation
Both LID and DIL were precisely weighed in quantities of 10 mg each and transferred into separate 10 ml volumetric flasks. They were dissolved with a small amount of acetonitrile, and additional acetonitrile was added to achieve a concentration of 1000 µg/ml for each drug individually. Further dilutions were carried out to prepare a mixture containing a concentration of 100 µg/ml for both LID and DIL, which served as the standard working solution.
2.5 Calibration curve determination
Using a Hamilton syringe through the CAMAG Linomat 5 sample applicator, 4 µl, 6 µl, 8 µl, 10 µl, and 12 µl volumes were applied from the standard working solution onto HPTLC silica gel aluminium plates (G60-F254) to construct the calibration curve.
2.6 Assay of marketed formulation
An amount equivalent to 10 mg of LID and DIL from the gel formulation was accurately weighed and transferred into a 10 ml volumetric flask, followed by the addition of a few millilitres of acetonitrile. The mixture was vortexed for ten minutes and then sonicated for another ten minutes. Subsequently, the mixture was filtered through Whatman filter paper No. 45. Acetonitrile was added to achieve a concentration of 1000 µg/ml for both LID and DIL. Further dilution was performed to obtain a concentration of 100 µg/ml of LID and DIL. From the 100 µg/ml solution, 8 µl was applied onto an HPTLC plate using a Hamilton syringe to achieve a concentration of 800 ng/band for LID and DIL. The chromatographic development was carried out up to 85 mm. After development, the plate was removed from the chamber and dried to remove the solvent. Densitometric scanning of the plate was performed at a speed of 20 mm/s using a TLC scanner. The quantities of LID and DIL in the formulation were calculated using regression equations.
2.7 Validation
The developed HPTLC method was validated according to International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) Q2 (R1) guideline.
2.7.1 Linearity
For calibration curve, the concentrations of 400 ng/band, 600 ng/band, 800 ng/band, 1000 ng/band, and 1200 ng/band were used to ascertain the linear relationship. Plotting peak area vs. concentration revealed a straight-line equation.
2.7.2 Precision
Precision is the degree of accuracy in measurements made from a homogeneous sample. Whereas interday denotes three determinations on separate days, intraday denotes three determinations on the same day. Three concentrations from the linearity range—the lowest, medium, and highest—400 ng/band, 800 ng/band, and 1200 ng/band—were chosen, and calculations were made for intermediate precision. To determine whether or not the procedure is repeatable, 800 ng/band of LID and DIL were used for injection and scanning repeatability. Using the obtained peak areas, the percentage RSD values were computed.
2.7.3 Accuracy
Accuracy is indicated by how closely measured values match their true values. The standard spiking method, in which the standard solutions of LID and DIL were spiked at three levels—80%, 100%, and 120%—is widely used to verify the correctness of the approach. The accuracy was conducted in triplicate, and the percentage of recovery was calculated using the mean of the peak areas.
2.7.4 Limit of detection and quantification
The term “limit of detection” refers to the lowest concentration at which an analyte within a matrix may be detected without quantification. The term "limit of quantification" refers to the lowest concentration at which the analyte may be detected inside the matrix while still being precisely and accurately quantified. The equation used to calculate LOD & LOQ are as shown below.
where σ is the standard deviation of y- intercepts of regression lines and S is the average slope of calibration curves.
2.7.5 Robustness
Intentionally made minor adjustments to optimal parameters, including run distance, wavelength, chamber saturation time, and mobile phase composition, were made, and results were noted. The method's robustness was evaluated in triplicate at 800 ng/band concentration for both LID and DIL. %RSD values were calculated from mean peak areas.
2.7.6 System suitability
An important part of the method development is the suitability testing of the system to ensure that it is suitable for performing LID and DIL analyses. Before each validation cycle, a system suitability test was performed on the chromatography system. Six identical standard solutions were injected to ensure system suitability. Retention factor (Rf), peak purity and resolution were obtained for each of the six appropriate injections.
2.7.7 Solution stability
Standard solutions were store in refrigerator and estimated to determine stability of solutions of LID and DIL.
3 Results and discussion
3.1 Wavelength selection
The sensitivity of the approach depends on the choice of an appropriate detection wavelength. Standard solutions of LID and DIL (10 µg/ml) were scanned in the UV range of 400–200 nm. UV spectra revealed that both medications absorb noticeably at 220 nm and therefore chosen as the detection wavelength as shown in Fig. 2.
3.2 Chromatographic optimization
Pre-coated silica gel G60-F254 aluminium sheet as stationary phase was selected on which 8 mm band length of LID and DIL samples were applied. Various trials were performed to optimize mobile phase which provide good separation between LID and DIL. The final optimized mobile phase obtained was toluene: methanol: acetonitrile: two drops of ammonia (7:2:1 v/v/v). The mobile phase was prepared in volumetric flask by taking appropriate quantities, transferred into 10 × 10 cm camag twin through chamber and kept undisturbed for 30 min for chamber saturation before each chromatographic development. The mobile phase was allowed to migrate about 85 mm distance on HPTLC plate. Then plates are removed and dried to evaporate solvents. Plate was scanned applying 6 × 0.45 mm slit dimension and at speed of 20 mm/s. These optimized conditions gave symmetrical and selective peak for LID at Rf 0.59 and DIL at Rf 0.48 as shown in Fig. 3.
3.3 Validation
ICH Q2 (R1) guideline was followed to validate the developed HPTLC method for quantification of LID and DIL simultaneously in bulk and gel dosage form. The results are mentioned and discussed below;
3.3.1 Linearity
LID and DIL were found to be linear within the specified concentration range of 400 ng/band to 1200 ng/band by developed HPTLC method. The overlay of the densitogram shown in Fig. 4. The Table 1 represents the regression analysis result.
3.3.2 Precision
% RSD values for LID and DIL were found to be 0.12–0.47% and 0.36–0.58%, respectively for intraday precision and % RSD values for LID and DIL were found to be 0.16–0.70% and 0.47–0.80%, respectively for interday precision. Lower %RSD values indicate that method is precise for quantifying LID and DIL simultaneously. %RSD for scanning and injection repeatability study was found to be less than 2%. Table 2 displays the result of precision study.
3.3.3 Accuracy
Accuracy study was performed using standard spiking method and % recoveries for LID and DIL were found to be 99.15–101.27% and 99.02–100.59%, respectively. The % recoveries for both the drugs were between the range of 98–102% indicate that the method is accurate. Result of accuracy data has been shown in Table 3.
3.3.4 Limit of detection and limit of quantification
LOD values for LID and DIL were found to be 28.93 ng/band and 18.72 ng/band, respectively. LOQ values for LID and DIL were found to be 87.67 ng/band and 56.74 ng/band, respectively. Lower values indicate that the method is highly sensitive for both detection and quantification of LID and DIL in small quantities.
3.3.5 Robustness
Minor changes in the chamber saturation time, run distance, wavelength, and mobile phase composition were employed for robustness study. For both LID and DIL, no significant change in the Rf values or the bands' compactness were observed. The robustness of method was assessed in triplicate manner at concentration of 800 ng/band for both LID and DIL. % RSD values were found to be less than 2 and data has been shown in Table 4. Thes summary of validation parameters has been shown in Table 5 for the developed HPTLC method.
3.3.6 System suitability parameters
Resolution between LID and DIL was found to be 2.5. Table 6 represents data obtained from system suitability test.
3.3.7 Solution stability
The solution stability data has been shown in Table 7 and it indicates that solutions of LID and DIL were found to be stable up to 24 h.
3.4 Assay of marketed formulation
The presented method was applied for marketed gel formulation to determine LID and DIL, qualitatively as well as quantitatively. The percentage purity for LID was found to be 99.98% ± 0.28 and the percentage purity for DIL was found to be 99.97% ± 1.40. Figure 5 shown the specificity of LID and DIL. Peak purity spectra of LID and DIL has been shown in Fig. 6. The assay result has been shown in Table 8.
4 Conclusion
The HPTLC method presented in this study offers an advanced yet cost-effective chromatographic approach for the simultaneous determination of LID and DIL. It utilized pre-coated silica gel G60-F254 aluminium sheets as the stationary phase and a mobile phase consisting of toluene: methanol: acetate: 2 drops of ammonia (7:2:1%v/v/v). Detection for both drugs was carried out at 220 nm. The method demonstrated linearity over a concentration range of 400–1200 ng/band for both LID and DIL. The limit of quantitation (LOQ) was determined to be 87.67 ng/band for LID and 56.74 ng/band for DIL. % Recovery studies showed excellent results with LID recovering between 99.15% and 101.27% and DIL recovering between 99.02% and 100.59%. Key advantages of this HPTLC method include its efficiency in solvent usage, allowing multiple analyses in a single run, and its cost-effectiveness compared to traditional liquid chromatographic methods. Furthermore, the method was validated to be precise, accurate, and robust, demonstrating its suitability for the quantitative analysis of LID and DIL in both bulk form and pharmaceutical gel dosage forms. In conclusion, the presented HPTLC method offers a reliable analytical tool for the quantification of LID and DIL, catering to both research and quality control applications in pharmaceutical analysis. Its robust performance and economical advantages make it a practical choice in analytical laboratories.
Data availability
Data has been provided within the manuscript and can be available on request basis.
References
PubChem, Lidocaine HCl. https://pubchem.ncbi.nlm.nih.gov/compound/Lidocaine-Hydrochloride (Accessed on January 2024).
PubChem, Diltiazem HCl. https://pubchem.ncbi.nlm.nih.gov/compound/Diltiazem-Hydrochloride (Accessed on January 2024).
A brief clinical summary of lidocaine HCl and Diltiazem HCl. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4755763/ (Accessed on April 2024).
Ingle S, Birajdar A, Matole V, Adlinge S, Nangare G. UV spectrophotometric method development and validation of lignocaine hydrochloride in bulk and semisolid dosage form. Res J Pharm Technol. 2021;14:5280–2.
Omer LS, Ali RJ. Extraction spectrophotometric determination of lidocaine hydrochloride in pharmaceuticals. Int J Chem. 2017;9:5539.
Bhusal P, Sharma M, Harrison J, Procter G, Andrews G, Jones D, Hill A, Svirskis D. Development, validation and application of a stability indicating HPLC method to quantify lidocaine from polyethylene-co-vinyl acetate (EVA) matrices and biological fluids. J Chromatogr Sci. 2017;55:832–8.
Hamdah MN, Matthew P, James SG, Dion RB. A high-performance liquid chromatography assay method for the determination of lidocaine in human serum. Pharm MDPI. 2017;9:52.
Salih MA, Aziz DF, Ali SI. Development and validation of new high performance liquid chromatography analytical technique for separation of diclofenac sodium and lidocaine hydrochloride from ampoule injection. Kurdistan J Appl Res. 2020;5:115–27.
Shaalan RA, Belal TS. HPLC–DAD stability indicating determination of nitrofurazone and lidocaine hydrochloride in their combined topical dosage form. J Chromatogr Sci. 2010;48:647–53.
Belal TS, Shaalan RA, Haggag RS. Gradient HPLC-diode array detector stability-indicating determination of lidocaine hydrochloride and cetylpyridinium chloride in two combined oral gel dosage forms. J AOAC Intern. 2011;94:503–12.
Doganay A, Koksel B, Gundogdu S, Capan Y. Simultaneous determination of dexpanthenol, lidocaine hydrochloride, mepyramine maleate and their related substances by Arp-hplc method in topical dosage forms. J Chromatogr Sci. 2018;56:903–11.
Al-Salman HN, Al-Jadaan SA, Alnuaim M, Hussein H. Estimation of lidocaine-HCl in pharmaceutical drugs by HPLC-UV system. Am J PharmTech Res. 2017;7:1–11.
Mohamed D, Kamal M. Enhanced HPLC-MS/MS method for the quantitative determination of the co-administered drugs ceftriaxone sodium and lidocaine hydrochloride in human plasma following an intramuscular injection and application to a pharmacokinetic study. Biomed Chromatogr. 2018;32:3422.
Suresha DN, Rashmi T, SenthilKumar GP. A novel method development and validation of deltiazem hydrochloride in pure and pharmaceutical dosage forms by using uv- spectrophotometric method. Am J PharmTech Res. 2019;9:226.
Nafisur R, Syed N. Spectrophotometric determination of diltiazem hydrochloride with sodium metavanadate. Microchem J. 2000;65:9–43.
Arafat M. Simple HPLC validated method for the determination of Diltiazem hydrochloride in human plasma. Int J Pharm Pharm Sci. 2014;6:213–6.
Patil BR, Bhusnure OG, Paul BN, Ghodke AY, Mulaje SS. Analytical method development and validation for the estimation of diltiazem hydrochloride in bulk and pharmaceutical dosage form by RP-HPLC. Int J Drug Regul Affairs. 2014;2:78–84.
Pawar RK, Lokhande R, Yadav R, Bind B, Velankar S. Development and validation of assay method by RP-HPLC for determination and quantitation of Diltiazem hydrochloride. Active Pharmaceutical Ingredient. 2017; 4.
Kulkarni AS, Jadhav SD, Khetmar SS, Bhatia MS. Development of chromatographic technique for simultaneous estimation of lovastatin and diltiazem hydrochloride. Mahidol Univ J Pharm Sci. 2012;39:17–23.
Mahajan N, Deshmukh S, Farooqui M. A novel stability-indicating method for known and unknown impurities profiling for diltiazem hydrochloride pharmaceutical dosage form (tablets). Future J Pharm Sci. 2017;7:1–13.
Devarajan PV, Dhavse VV. High-performance thin-layer chromatographic determination of diltiazem hydrochloride as bulk drug and in pharmaceutical preparations. J Chromatogr Biomed Sci Appl. 1998;706:362–6.
Chaudhari N, Saskshee S, Patel S, Chandwani J, Kachhiya H. A validated UV spectroscopic method for quantification of lidocaine HCl and diltiazem HCl. J Adv Res Rev Med Med. 2024;1:12–21.
ICH Guidelines Q2 (R2), “Validation of Analytical Procedure: Text and Methodology” Geneva, Switzerland, March, 2022.
Acknowledgements
The authors are extremely thankful to Indukaka Ipcowala College of Pharmacy, New Vallabh Vidyanagar, SICART and The Charutar Vidya Mandal University, Vallabh Vidyanagar for providing all necessary facilities to conduct the presented research work.
Funding
This research did not receive any specific grant from any funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Maitri Shah and Hetaben Kachhiya contributed to design of the research work and wrote the main manuscript. Jinal Tandel, Usmangani Chhalotiya and Dimal Shah prepared all figures and tables. Adarsh Patelia and Mehul Patel reviewed the tables and approved the version to be published. All the authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shah, M., Kachhiya, H., Tandel, J. et al. A validated HPTLC method for quantification of a new combination containing Lidocaine HCl and Diltiazem HCl. Discov. Chem. 1, 12 (2024). https://doi.org/10.1007/s44371-024-00014-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44371-024-00014-w