Abstract
A mortality of bees (Apis mellifera) caused by fipronil intoxication, due to its indiscriminate use in crops, has long been attracting the scientific community’s attention, either due to its acute or residual effects. In this study, we assessed the cardiac activity as a biomarker of fipronil intoxication through electrophysiological recordings of bees. Eighteen foragers, from the apiary of EMBRAPA—Eastern Amazon (Belém-Pará), bees were previously anesthetized at low temperature (− 10 °C) for 5 min and properly restrained and fixed on a stereotaxic base, where electrodes were implanted. All these procedures were carried out within a Faraday cage. Eighteen bees were used in the study. Worker bees engaged in foraging activities were selected. The bees were divided into a control group and a group treated with fipronil at 0.025 mg/bee (n = 9). The recordings lasted for 4 min and were evaluated at 1-s intervals represented by the following letters: A = (2–3 s), B = (59–60 s), C = (119–120 s), D = (179–180 s), and E = (239–240 s). The results showed that fipronil reduced the frequency and intensity of cardiac activity, exhibiting rapidly evolving effects, and promoting a disruption of homeostasis in bee hemodynamics. Through the obtained data, it was observed variation in spike amplitude, with a loss of cardiac strength and magnitude of the electrical impulse in the bee’s heart during exposure to fipronil.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Agriculture’s growth has resulted in increased use of insecticides to combat pests in crops, which have been intensively used for over half a century [1, 2]. The application of these products, using various methods, is widespread, particularly in systemic insecticides, with fipronil being one of its representatives [3]. This pesticide is a derivative of the fiprole family [4,5,6], widely used in cereal and vegetable crops [3, 7].
The effects exerted by fipronil are due to its systemic action related to antagonism in receptors present in chloride channels controlled by gamma-aminobutyric acid (GABA). It competes with GABA for binding sites and, by irreversibly binding to the receptor, prevents nerve impulse transmission, leading to inhibition of synaptic transmission. Additionally, in insects, fipronil can also bind to glutamate-controlled chloride channels, which may explain its differences in selectivity and toxicity in vertebrates and invertebrates [6, 8].
According to Zaluski [9], even at reduced doses, the chemical component in question reveals more severe behavioral alterations in bees through contact than ingestion, mainly due to the lack of enzyme detoxification mechanisms in the digestive system of insects, when contact occurs topically [10]. Holder et al. [11] found a high sensitivity of bees to the toxic effects of fipronil, especially due to its cumulative nature.
The importance of ecological balance, the management of cultivable plant resources, and measures enabling the discovery of appropriate conservation and management techniques that minimally affect insects with zootechnical potential are highlighted [12]. Bees are crucial in ecological systems for its role as pollinators, and as a source of income for beekeepers [13,14,15]. According to Michener [16], species in the Apini tribe, much like in the Meliponini one, build perennial colonies characterized by morphologically distinct female castes. These colonies reproduce through a process called fission, where the old queen and a group of workers depart to establish a new colony elsewhere. The size of these colonies varies, ranging from a few thousand to over 60,000 workers. However, habitat degradation and a lack of information on bee breeding and sustainable management, along with the effects of pesticides on colonies, are among the main challenges for mapping information as well as effective methodologies for preserving these species [17].
Fipronil is particularly toxic to some species of laboratory mammals upon oral exposure (LD50 = 97 mg/kg for rats; LD50 = 91 mg/kg for mice), but very toxic to bees (LD50 = 0.004 µg/bee) [18, 19]. The Pesticide Action Network [20] has compiled a list of banned pesticides, among which fipronil stands out. This compound has been banned for use and sale in 36 countries, including nations such as Cape Verde and Mauritania, located in Africa, as well as in the United Kingdom, Vietnam and 27 countries belonging to the European Union. A comprehensive analysis of publications related to this pesticide can provide valuable insights for new research projects, the development of detection and degradation methods, as well as guiding environmental policies [21, 22].
Although the literature presents robust data on cardiac function in other experimental models, studies on cardiac function and blood circulation (hemolymph) in bees are relatively scarce and have been carried out especially in the honey bee, Apis mellifera, and, to a lesser extent, in the bumble bee, Bombus terrestris. Most of these studies are descriptive, but more recent studies have focused on the effects of stressors, mainly pesticides, on the heart activity and other functions [23,24,25].
In view of the scarcity of articles elucidating these mechanisms, which remain obscure, the aim of this article was to describe the effect of fipronil on the cardiac activity of Apis mellifera foragers, evaluating the underlying mechanisms that may alter cardiac recordings.
2 Materials and methods
2.1 Studied species
Eighteen Apis mellifera workers were obtained from an apiary from EMBRAPA—Eastern Amazon (Belém-Pará-Brazil) (− 1.4338344019712814, − 48.44947809710463), which were returned to their nest after the experiments. The bees were transported to the Pharmacology and Toxicology of Natural Products Laboratory at the Federal University of Pará—Institute of Biological Sciences, where they were housed in a temperature-regulated environment (26–27 °C) and subjected to the experiments described below.
2.2 Preparation of bees for experiment
The bees were initially placed in a Petri dish (150 × 20 mm) and subjected to a temperature of − 10 °C for 5 min to reduce their activity and induce the loss of the laying reflex (Fig. 1A). Next, foragers were exposed to a temperature of 26 °C, the laying reflex recovery occurred with an average latency of 223 ± 68 s (Fig. 1B, C). This procedure was necessary to facilitate the fixation of bees on a polyethylene foam platform, with a small elastic band between the thorax and abdomen, and to facilitate electrode implantation (Fig. 2A). Pins were used to secure the bees to the foam and prevent movement. This study followed the method of Contrera et al. [26]. All recordings lasted 4 min, with an initial 2-min accommodation period.
Characteristics of Apis mellifera subjected to (− 10 °C) for 5 min, observing the loss of the laying reflex (indicated by the blue arrow from right to left) (A); the animal subjected to (26 °C) begins gradual recovery of reflexes with slow limb movements with a latency of 169 ± 23 s (B); followed by recovery of the laying reflex with a latency of 223 ± 68 s (C)
Stereotaxic coordinates used in animals for signal acquisition, positioning of electrodes spaced 1 mm apart, coupled to a high-impedance amplifier (50,000× signal amplification) (A); recording acquisition of recordings with graph elements showing the characteristic signal on a monitor (B); amplification of Apis mellifera recording, represented by 0.2 s of the trace demonstrating the parameters analyzed allowing the evaluation of Spike Frequency (SPM), Spike Amplitude (mV), Spike Interval (ms) and Spike Duration (ms) (C)
2.3 Electrode fabrication, implantation, and record acquisition
The electrodes were made from JST SM cables with 2-pin Jacks, each 13 cm in length. Nickel–chromium wire electrodes (Morelli Orthodontics), spaced 1 mm apart, with a diameter of 0.2 mm and a length of 2 mm, were insulated with liquid insulation from the brand Quimatic Tapmatic. After the material dried, the electrode was fixed in a stereotaxic device. After bee fixation, the following coordinates were observed, considering the recording electrode as a parameter: the zero point was at the thorax-abdomen intersection on the mid-sagittal line, with an anteroposterior coordinate of 1 mm and a dorsoventral coordinate of 0.6 mm (Fig. 2A). Graph elements were then recorded after the procedure. The entire recording acquisition process was conducted within a Faraday cage. The electrodes were connected to a high-impedance amplifier (Grass Technologies, P511) with a signal amplification of 50,000×, monitored by an oscilloscope (Protek, 6510) (Fig. 2B, C). After being fixed, the bees were exposed to 0.2 mL of a 0.25 mg/mL solution on the animal’s back of the insecticide fipronil in spray (30 mL) of the brand formilix from the company formilix Indústria e Comércio located in the municipality of Ibicui, Bahia-Brazil.
All recordings had a duration of 4 min. Fragments were extracted from each recording every minute for analysis and comparison of the effects of Fipronil (0.25 mg/mL) on cardiac activity. Thus, we analyzed the last second of cardiac activity in five different periods: A = 2–3 s (initial period), B = 59–60 s, C = 119–120 s, D = 179–180 s, and E = 239–240 s. Therefore, the experiment consisted of two groups: the control group (n = 9) and the treated group (n = 9) with Fipronil 0.25 mg/mL in a volume of 0.1 mL (0.025 mg/bee), in which the analyzed period was indicated by the letter F (AF, BF, CF, DF, and EF). This period delineation was necessary due to the extensive dataset generated, which would be challenging to visualize if presented continuously. The mean latency for tremors and movement incoordination after fipronil application was 23.14 ± 9.3 s.
2.4 Statistical analyses
The power of record fragments, Spike Frequency (SPM), Amplitude (mV), Spike Interval (ms), and Spike Duration (ms) (Fig. 2B, C) were subjected to tests of normality and homogeneity of variance to assess data variations using the Kolmogorov–Smirnov and Levene tests, respectively. Summarized data are presented as mean ± standard deviation (SD), and p-values are provided. Comparisons between periods were based on two-way ANOVA followed by Tukey’s test for multiple comparisons. Statistical analyses and graph constructions were performed using GraphPad Prism, version 8 (Graph-Pad Software Inc., San Diego, CA, USA).
3 Results
In the control group, upon analyzing the records with a duration of 4 min, the average spike frequency was observed to be 1333 ± 91.47 Spikes per minute (SPM), with an average spike amplitude of 1.575 ± 0.3383 mV. The intervals between spikes had an average of 44.56 ± 3.552 ms, and the duration of the spikes was 8.262 ± 1.184 ms. This indicates a sustained rhythm with repetitions of morphographic elements, showing minimal variations (Table 1).
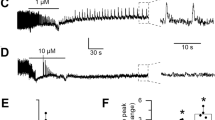
The 4-min records analyzed in A. mellifera showed variations in recording amplitude when comparing the control group (Fig. 3A) and the Fipronil-treated group (Fig. 3B). Based on morphographic elements captured by the electrode, larger irregularities can be observed in the records of the Fipronil-treated group (Fig. 3A, B). Fragments were extracted from each record for analysis at the end of each minute of recording and were used to compare power between the groups. During the initial records (2–3 s), the mean powers were similar between the control group (40.53 ± 2.289 mV2/Hz × 10–3) and the fipronil-treated group (35.41 ± 3.633 mV2/Hz × 10–3) (p = 0.0715). At the end of the first minute (59–60 s) after Fipronil application, the power of the cardiac record decreased compared to the control (24.75 ± 3.748 mV2/Hz × 10–3 vs 33.61 ± 5.125 mV2/Hz × 10–3). At the end of the second minute (119–120 s), the power of the record decreased further (24.68 ± 4.978 mV2/Hz × 10–3) compared to the control (33.22 ± 3.289 mV2/Hz × 10–3). At the end of the third minute (179–180 s) of Fipronil contact, the recorded power (9.396 ± 2.120 mV2/Hz × 10–3) was lower than the control group average (27.46 ± 3.627 mV2/Hz × 10–3). By the end of the fourth minute of records, the Fipronil-treated group showed an average power of (8.739 ± 0.834 mV2/Hz × 10–3), which was lower than the control group (27.40 ± 2.2909 mV2/Hz × 10–3) (Fig. 3C).
Tracings represented by 4 min of recording, demonstrating the areas analyzed (dotted in red): A = (2–3 s), B = (59–60 s), C = (119–120 s), D = (179–180 s), and E = (239–240 s) (A); tracings recorded in bees in contact with Fipronil 0.25 mg/mL (red dotted): AF = (2–3 s), BF = (59–60 s), CF = (119–120 s), DF = (179–180 s), and EF = (239–240 s) (B); the linear power graph of the recorded activity for the control and Fipronil-treated groups in Apis mellifera (C). (After ANOVA followed by Tukey; *p < 0.001), (n = 9)
For each change in the trace for the control group and the Fipronil-treated group, a pattern of behavior of the recorded graph elements was analyzed over the contact time, always in the final second of each minute: 2–3 s, 59–60 s, 119–120 s, 179–180 s, and 239–240 s. For each record, greater variations in the recorded graph elements can be observed for the Fipronil-treated group (Fig. 4A–E). Therefore, the frequency of spikes in period A was similar between the control (1227 ± 82.48 SPM) and the experimental groups (1303 ± 48.48 SPM) (p = 0.3224). In period B, there was an increase in heart rate for the Fipronil-treated group (1627 ± 85.17 SPM) compared to the control (1379 ± 41.70 SPM). The increased spike frequency for the treated group was maintained in period C with an average of (1754 ± 55.70 SPM) vs. Control (1427 ± 67.83 SPM). In period D, the treated group showed a decrease in spiking frequency (504.4 ± 76.67 SPM) compared to the control (1286 ± 47.53 SPM), and this condition was maintained in period E, with the treated group showing a decrease in spiking frequency (513 ± 81.09 SPM) compared to the control group (1346 ± 55.25 SPM) (Fig. 5A).
Evaluation of the recorded activity of Apis mellifera for the control and fipronil-treated groups. In each trace, Spike Frequency (SPM), Spike Amplitude (mV), Interval between Spikes (ms), and Spike Duration (ms) were analyzed. After ANOVA followed by Tukey’s test. *p < 0.05, **p < 0.01, ***p < 0.001 (n = 9)
The amplitude of the spikes in period A for the Fipronil-treated group (2.503 ± 0.617 mV) was higher than for the control group (1.321 ± 0.159 mV), demonstrating premature alteration of the recorded activity. In period B, the treated group showed a similar mean (2.161 ± 0.492 mV) compared to the control group (1.766 ± 0.256 mV) (p = 0.413), and this relationship was similar for period C, with the mean for the treated group (2.230 ± 0.2324 mV) vs the control group (1.754 ± 0.484 mV) (p = 0.1739). In period D, the control group (1.485 ± 0.277 mV) was higher than the treated group (0.9176 ± 0.213 mV), and in period E, the difference persisted between the control group (1.547 ± 0.257 mV) and the treated group (0.9311 ± 0.143 mV) (Fig. 5B).
For the interval between spikes, there were no significant differences for periods A between the control group (48.11 ± 3.65 ms) and the treated group (45.11 ± 1.45 ms) (p = 0.988). For period B, the control group (42.78 ± 1.39 ms) and the treated group (36.56 ± 2.007 ms) did not show significant differences (p = 0.496). The same relationship occurred in period C, with the mean for the control group (41.11 ± 3.48 ms) vs. the treated group (33.78 ± 1.39 ms) (p = 0.265). However, the interval between spikes increased in the treated group in periods D (115.1 ± 13.49 ms) and E (116.9 ± 12.31 ms) compared to period D of the control (46.44 ± 2.007 ms) and period E (44.33 ± 1.658 ms) (Fig. 5C).
During the recording period, the duration of the spike in period A for the control group (9.48 ± 1.41 ms) was similar to the treated group (9.23 ± 1.19 ms) (p = 0.999). For period B, the control group (8.50 ± 0.65 ms) was greater than the treated group (5.77 ± 0.89 ms). For period C, the control group (8.32 ± 1.01 ms) was greater than the treated group (6.333 ± 1.11 ms), and the same occurred for periods D and E, where the control group (7.55 ± 0.79 ms) and (7.44 ± 0.707 ms) was greater than the treated group (5.11 ± 0.92 ms) and (4.66 ± 0.701 ms) (Fig. 5D).
4 Discussion
Records of Apis mellifera cardiac activity have been sparsely reported [27, 28], and the impact of the toxic agent fipronil on assessing the disruption of homeostasis in bee hemodynamics after contact evolves rapidly. Studies demonstrate that the effectiveness of fipronil against the tick, Rhipicephalus sanguineus, lasts for 42 days after treatment [29]. This prolonged residual activity should be considered, particularly for more susceptible insect species such as bees.
Fipronil is a widely used pesticide in agriculture for pest control, but, in 2022, the Pesticide Action Network (PAN) pointed out that fipronil was among the pesticides banned in 36 countries. These countries include European Union nations such as the United Kingdom and other regions such as Cape Verde, Mauritania, and Vietnam [22]. These bans were implemented as part of efforts to reduce the risks associated with the use of this pesticide. However, it is associated with the widespread death of pollinating insects [3, 30, 31], as well as systemic effects. It primarily antagonizes GABAA-type chloride channels in invertebrate nerve endings, resulting in a state of excessive stimulation often associated with seizures, ultimately leading to death [32, 33]. After the application of fipronil, the bees exhibited tremors and motor in coordination with a latency of 23.14 ± 9.3 s, indicating rapid absorption and manifestation of effects.
All bee species are susceptible to the neurotoxic mechanisms of fipronil [34]. It is important to emphasize that native stingless bees are also affected by this toxicity, causing functionally detrimental events in various systems, including the endocrine system [35], digestive system [36] and especially the circulatory system. In cardiac activity records, a decrease in signal power occurred over 4 min of recording, intensifying over time with a reduction in power more than three times compared to the control group.
Intoxication by GABAergic neurotransmitter antagonists can induce seizures, and during convulsive episodes, changes in cardiac functionality can occur in various species [37,38,39,40,41,42]. The records demonstrated an initial momentary increase in the frequency of Spikes followed by a persistent decrease and an increase in irregularities in the graph elements, suggesting arrhythmias. The pattern of heart rate variability caused by fipronil appears to be related to a convulsive crisis associated with damage to the neuronal pathway that regulates cardiac activity [9, 43,44,45,46,47].
In vertebrates, these changes may occur due to the impairment of brain structures that regulate autonomic control, such as the hippocampus, entorhinal cortex, and amygdala [48]. However, in invertebrates, changes found in the hearts of cockroaches of the species Nauphoeta cinerea, in a semi-isolated preparation, from exposure to sublethal doses of fipronil also showed a negative chronotropic effect of this compound [49]. Even with extensive washing of the preparations, the heart rate did not recover, suggesting the irreversible nature of these cardiac damages according to Michener [16]. Changes in cardiac activity, such as a rapid decrease in spike amplitude and frequency, indicate that fipronil has an affinity for bee cardiac cells. The neurological effects caused by fipronil may cause secondary damage to other systems, and by ECG analysis, it could be evaluated the harmful impact of this compound on the species of bees studied. However, further studies are needed to define the mechanism of action linked to fipronil on the cardiac activity of A. mellifera. Nevertheless, through electrophysiological tools, it was observed variations in spike amplitude, loss of cardiac force, and magnitude of electrical impulse in the bee’s heart during fipronil exposure.
Our results point to the real need for new studies to be carried out so that mechanisms of fipronil action in bees are understood. It includes investigating the neural components involved in regulating the heart rate and directing the cardiac function, as well as exploring the influence of various ion channels, cardiomodulatory peptides and neurotransmitters. It is also necessary to clarify whether the origin of this insect’s heartbeat is myogenic, originating within the heart muscle itself, or neurogenic, originating from neural control over the insect’s cardiac function and heart rate modulation. By delving deeper into these questions, we can unlock valuable insights into the interaction between physiological processes in bees, thus advancing our understanding of their cardiovascular systems and the maintenance of homeostasis.
Data availability
Data will be made available on request.
Code availability
Not applicable.
References
Chaves GP, Magalhães SB. Peasants and agro-chemicals in eastern Amazonia. Dev Environ. 2021;58:63–81. https://doi.org/10.5380/dma.v58i0.73069.
Potts SG, Imperatriz-Fonseca V, Ngo HT, Aizen MA, Biesmeijer JC, Breeze TD, Dicks LV, Garibaldi LA, Hill R, Settele J, Vanbergen AJ. Safeguarding pollinators and their values to human well-being. Nature. 2016;540(7632):220–9. https://doi.org/10.1038/nature20588.
Bonmatin JM, Giorio C, Girolami V, Goulson D, Kreutzweiser DP, Krupke C, Liess M, Long E, Marzaro M, Mitchell EA, Noome DA, Simon-Delso N, Tapparo A. Environmental fate and exposure; neonicotinoids and fipronil. Environ Sci Pollut Res. 2015;22:35–67. https://doi.org/10.1007/s11356-014-3332-7.
Magalhães JZ, Sandini TM, Udo MSB, Fukushima AR, de Souza Spinosa H. Fipronil: usos, características farmacológicas e toxicológicas. Revista Intertox de Toxicologia, Risco Ambiental e Sociedade. 2018. https://doi.org/10.22280/revintervol11ed1.344.
Papa G, Maier R, Durazzo A, Lucarini M, Karabagias IK, Plutino M, Bianchetto E, Aromolo R, Pignatti G, Ambrogio A, Pellecchia M, Negri I. The honey bee Apis mellifera: an insect at the interface between human and ecosystem health. Biology. 2022;11(2):233. https://doi.org/10.3390/biology11020233.
Sánchez-Bayo F, Tennekes HA, Goka K. Impact of systemic insecticides on organisms and ecosystems. In: Insecticides-development of safer and more effective technologies. Rijeka: IntechOpen; 2013. p. 365–414. https://doi.org/10.5772/52831.
Badgujar PC, Pawar NN, Chandratre GA, Telang AG, Sharma AK. Fipronil induced oxidative stress in kidney and brain of mice: protective effect of vitamin E and vitamin C. Pestic Biochem Physiol. 2015;118:10–8. https://doi.org/10.1016/j.pestbp.2014.10.013.
Narahashi T, Zhao X, Ikeda T, Nagata K, Yeh JZ. Differential actions of insecticides on target sites: basis for selective toxicity. Hum Exp Toxicol. 2007;26(4):361–6. https://doi.org/10.1177/0960327106078408.
Zaluski R, Kadri SM, Alonso DP, Martins Ribolla PE, de Oliveira OR. Fipronil promotes motor and behavioral changes in honey bees (Apis mellifera) and affects the development of colonies exposed to sublethal doses. Environ Toxicol Chem. 2015;34(5):1062–9. https://doi.org/10.1002/etc.2889. (Epub 2015 Mar 30).
Miranda JE, et al. Susceptibility of Apis mellifera (Hymenoptera: Apidae) to pellitorine, na amide isolated from Piper tuberculatum (Piperaceae). Apidologie. 2003;34(4):409–15. https://doi.org/10.1051/apido:2003036.
Holder PJ, Jones A, Tyler CR, Cresswell JE. Fipronil pesticide as a suspect in historical mass mortalities of honey bees. Proc Natl Acad Sci USA. 2018;115(51):13033–8. https://doi.org/10.1073/pnas.1804934115. (Epub 2018 Dec 3).
Barbosa DB, Crupinski EF, Silveira RN, Limberger DCH. Bees and their ecosystem service of pollination. Ver Elet Cient UERGS. 2017;3(4):694–703. https://doi.org/10.21674/2448-0479.34.694-703.
Dicks, LV, Breeze, TD, Ngo, HT et al. A global-scale expert assessment of drivers and risks associated with pollinator decline. Nat Ecol Evol 2021;5:1453–1461. https://doi.org/10.1038/s41559-021-01534-9.
Faita MR, Chaves A, Nodari RO. The expansion of agribusiness: harmful impacts of deforestation, pesticides and transgenics on bees. Desenvolv Meio Ambiente. 2021;57:79–105. https://doi.org/10.5380/dma.v56i0.76157.
Patel V, Pauli N, Biggs E, et al. Why bees are critical for achieving sustainable development. Ambio. 2021;50:49–59. https://doi.org/10.1007/s13280-020-01333-9.
Michener CD. Lisotrigona na Tailândia e o macho do gênero (Hymenoptera: Apidae: Meliponini). J Kans Entomol Soc. 2007;80(2):130–5. https://doi.org/10.2317/0022-8567(2007)80[130:LITATM]2.0.CO;2.
Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE. Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol. 2010;25(6):345–53. https://doi.org/10.1016/j.tree.2010.01.007.
Bovi TS, Zaluski R, Orsi RO. Toxicity and motor changes in Africanized honey bees (Apis mellifera L.) exposed to fipronil and imidacloprid. An Acad Bras Cienc. 2018;90(1):239–45. https://doi.org/10.1590/0001-3765201820150191.
Tingle CC, Rother JA, Dewhurst CF, Lauer S, King WJ. Fipronil: environmental fate, ecotoxicology, and human health concerns. Rev Environ Contam Toxicol. 2003;176:1–66. https://doi.org/10.1007/978-1-4899-7283-5_1.
PAN Pesticide Action Network. PAN international consolidated list of banned pesticides. Pesticide Action Network. 2022. https://pan-international.org/pan-international-consolidated-list-of-banned-pesticides/. Accessed 07 May 2024.
Gonçalves S, Vasconcelos MW, Mota TFM, Lopes JMH, Guimaraes LJ, Miglioranza KSB, Ghisi NC. Identifying global trends and gaps in research on pesticide fipronil: a scientometric review. Environ Sci Pollut Res Int. 2022;29(52):79111–25. https://doi.org/10.1007/s11356-022-21135-8. (Epub 2022 Jun 15).
Liang S-X, Zhao Z, Fan C-L, Jian-zhong Xu, Li H, Chang Q-Y, Pang G-F. Fipronil residues and risk assessment of Chinese marketed fruits and vegetables: a long-term investigation over 6 years. Food Control. 2019;106: 106734. https://doi.org/10.1016/j.foodcont.2019.106734.
O’Neal S, Anderson T. Dissection and observation of honey bee dorsal vessel for studies cardiac function. J Vis Exp. 2016;118:55029.
O’Neal S, Brewster CC, Bloomquist JF, Anderson T. Amitraz and its metabolite modulate honey bee cardiac function and tolerance to viral infection. J Invertebr Pathol. 2017;149:119–26.
Yelkovan S, Arıkan H, Çakıcı Ö. Caste and age related changes in circulatory hemocytes of honey bee, Apis mellifera anatolica (Hymenoptera: Apidae). J Apic Res. 2021;60:512–21.
Contrera FAL, Lopes BDSC, Paz CAD, Hamoy MKO, Santos MFD, Barbosa GB, Amaral ALGD, Pinho LHB, Hamoy M. First records of heartbeats via ECG in a stingless bee, Melipona flavolineata (Apidae, Meliponini), during contention stress using isoflurane as an anesthetic. Insects. 2023;14(8):696. https://doi.org/10.3390/insects14080696.
Papaefthimiou C, Papachristoforou A, Theophilidis G. Biphasic responses of the honeybee heart to nanomolar concentrations of amitraz. Pestic Biochem Physiol. 2013;107:132–7.
Schwab ER, Chilson RA, Eddleman CD. Heartbeat rate modulation mediated by the ventral nerve cord in the honeybee, Apis mellifera. J Comp Physiol B. 1991;161:602–10.
Fischer VL, Heidmann MJ, Faria EF, Rizzi VG, Bragaglia GN, Nascimento CG, Castro BG. Acaricidal efficacy of topical formulation of fipronil in naturally infested dogs in Amazonic region, Brazil. Rev Bras Parasitol Vet. 2013;22(1):186–8. https://doi.org/10.1590/s1984-29612013000100037.
Kairo G, Poquet Y, Haji H, Tchamitchian S, Cousin M, Bonnet M, Pelissier M, Kretzschmar A, Belzunces LP, Brunet J-L. Evaluation of the toxic effect of pesticides on the fertility of honey bee drones using laboratory and semi-field approaches: a case study of fipronil. Environ Toxicol Chem. 2017;36:l2345-2351. https://doi.org/10.1002/etc.3773.
Zaluski R, Justulin LA Jr, Orsi RDO. Field-relevant doses of the systemic insecticide fipronil and fungicide pyraclostrobin impair mandibular and hypopharyngeal glands in nurse honeybees (Apis mellifera). Sci Rep. 2017;7(1):15217. https://doi.org/10.1038/s41598-017-15581-5.
Aidley DJ, Stanfield PR. Ion channels: molecules in action. New York: Cambridge University Press; 1996.
Ratra GS, Casida JE. GABA receptor subunit composition relative to insecticide potency and selectivity. Toxicol Lett. 2001;122(3):215–22. https://doi.org/10.1016/s0378-4274(01)00366-6.
Casida JE, Durkin KA. Neuroactive insecticides: targets, selectivity, resistance, and secondary. Annu Rev Entomol. 2013;58:99–117. https://doi.org/10.1146/annurev-ento-120811-153645.
Barbosa WF, Tomé HV, Bernardes RC, Siqueira MA, Smagghe G, Guedes RN. Biopesticide-induced behavioral and morphological alterations in the stingless bee Melipona quadrifasciata. Environ Toxicol Chem. 2015;34(9):2149–58. https://doi.org/10.1002/etc.3053. (Epub 2015 Jul 30).
Cruz AS, da Silva-Zacarin ECM, Bueno OC, et al. Morphological alterations induced by boric acid and fipronil in the midgut of worker honeybee (Apis mellifera L.) larvae. Cell Biol Toxicol. 2010;26:165–76. https://doi.org/10.1007/s10565-009-9126-x.
Hamoy AO, Fonseca SMD, Cei GL, Júnior FLDA, Hamoy MKO, Ribeiro RM, Barbas LA, Muto N, Hamoy M. Behavioral, electrocorticographic and electrocardiologic changes in Colossoma macropomum (Tambaqui) in the effect of cunaniol. PLoS ONE. 2023;18(6): e0287681. https://doi.org/10.1371/journal.pone.0287681.
Hotta H, Koizumi K, Stewart M. Cardiac sympathetic nerve activity during kainic acid-induced limbic cortical seizures in rats. Epilepsia. 2009;50(4):923–7. https://doi.org/10.1111/j.1528-1167.2008.01860.x. (Epub 2008 Dec 4).
Mazzola L, Mauguière F, Chouchou F. Central control of cardiac activity as assessed by intra-cerebral recordings and stimulations. Neurophysiol Clin. 2023;53(2): 102849. https://doi.org/10.1016/j.neucli.2023.102849. (Epub 2023 Mar 1).
Nagata S, Fujiwara K, Kuga K, Ozaki H. Prediction of GABA receptor antagonist-induced convulsion in cynomolgus monkeys by combining machine learning and heart rate variability analysis. J Pharmacol Toxicol Methods. 2021;112: 107127. https://doi.org/10.1016/j.vascn.2021.107127. (Epub 2021 Oct 6).
Naggar I, Sakamoto K, Jones S, Stewart M. Autonomic nerve activity and cardiovascular changes during discrete seizures in rats. Auton Neurosci. 2022;2022(240): 102971. https://doi.org/10.1016/j.autneu.2022.102971. (Epub 2022 Mar 16).
Sakamoto K, Saito T, Orman R, Koizumi K, Lazar J, Salciccioli L, Stewart M. Autonomic consequences of kainic acid-induced limbic cortical seizures in rats: peripheral autonomic nerve activity, acute cardiovascular changes, and death. Epilepsia. 2008;49(6):982–96. https://doi.org/10.1111/j.1528-1167.2008.01545.x. (Epub 2008 Mar 5).
Bharathraj MY, Venugopal K, Jaligidad K, Karibasappa H, Kumar H. Fipronil compound consumption presenting as status epilepticus. Toxicol Int. 2015;22(1):165–6. https://doi.org/10.4103/0971-6580.172280.
d’Ovidio D, Cortellini S. Successful management of fipronil toxicosis in two pet rabbits. Open Vet J. 2022;12(4):508–10. https://doi.org/10.5455/OVJ.2022.v12.i4.13. (Epub 2022 Aug 5).
Fung HT, Chan KK, Ching WM, Kam CW. A case of accidental ingestion of ant bait containing fipronil. J Toxicol Clin Toxicol. 2003;41(3):245–8. https://doi.org/10.1081/clt-120021106.
Jayaprakash R, Elangovan A, Nagaraju P. Fipronil and acetamiprid poisoning: new perils. Indian J Crit Care Med. 2022;26(4):526–7. https://doi.org/10.5005/jp-journals-10071-24205.
Li P, Akk G. The insecticide fipronil and its metabolite fipronil sulphone inhibit the rat α1β2γ2L GABA(A) receptor. Br J Pharmacol. 2008;155(5):783–94. https://doi.org/10.1038/bjp.2008.309. (Epub 2008 Jul 28).
Bernasconi N, Natsume J, Bernasconi A. Progression in temporal lobe epilepsy: differential atrophy in mesial temporal structures. Neurology. 2005;65(2):223–8. https://doi.org/10.1212/01.wnl.0000169066.46912.fa.
Rosa ME, Campos L, Borges BT, Santos S, Barreto YC, de Assis DR, Hyslop S, de Souza VQ, Vinadé L, Dal Belo CA. Fipronil affects cockroach behavior and olfactory memory. J Exp Biol. 2023;226(8): jeb245239. https://doi.org/10.1242/jeb.245239. (Epub 2023 Apr 21).
Acknowledgements
We thank the National Council for Scientific and Technological Development (CNPq) for the productivity grant to Felipe Contrera (process 310112/2022-2) and the Coordination for the Improvement of Higher Education Personnel (CAPES, Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior—Finance Code 001) for a Ph.D scholarship to Clarissa Paz (process number 88887.800862/2023-00).
Funding
We thank the National Council for Scientific and Technological Development (CNPq) for the productivity grant to Felipe Contrera (process 310112/2022-2) and the Coordination for the Improvement of Higher Education Personnel (CAPES, Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior—Finance Code 001) for a Ph.D scholarship to Clarissa Paz (process number 88887.800862/2023-00).
Author information
Authors and Affiliations
Contributions
C.A.P., F.A.L.C.; M.H.: conceptualization. C.A.P., F.A.L.C.; L.E.Q., D.B.A.: data curation. L.V.d.S., M.F.d.S., R.d.C.F., Y.d.S.D., A.P.L.R., D.S.P., F.A.L.C., N.A.M., G.B.B., T.M.C., A.L.C.C., R.N.O.S., P.F.P.H.: methodology. C.A.P., F.A.L.C.; L.E.Q., D.B.A., N.A.M., G.B.B., T.M.C., A.L.C.C., R.N.O.S., P.F.P.H., M.K.O.H.: formal analysis, writing—original draft preparation, writing—reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
da Paz, C.A., Eiró-Quirino, L., de Araújo, D.B. et al. Changes recorded in cardiac graphoelements of bees (Apis mellifera) during contact with fipronil. Discov Anim 1, 16 (2024). https://doi.org/10.1007/s44338-024-00017-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44338-024-00017-y