Abstract
The livestock industry faces challenges such as limited protein feed resources, suboptimal nitrogen utilization efficiency, and environmental impacts. Reducing the crude protein content in piglet diets has been shown to alleviate these issues, offering benefits to both animal welfare and environmental sustainability. However, low-protein (LP) diets can negatively affect piglet health and growth, necessitating strategies to mitigate these effects. The study aimed to explore the effects of supplementing LP diets with N-acetylglutamate (NAG) and Patchouli (PTC) on the health and growth of weaned piglets, focusing on growth metrics, antioxidant capabilities, intestinal barrier integrity, and inflammatory response. One hundred twenty healthy piglets were randomly assigned to five dietary groups, including a standard control diet (CON), an LP diet, and LP diets supplemented with NAG, PTC, or both. The trial lasted four weeks, and the piglets' growth, immune response, antioxidant status, and intestinal health were assessed. Piglets on the LP diet had lower final body weights and average daily weight gains. However, supplementation with NAG and PTC improved antioxidant defense, reduced inflammation, and enhanced intestinal health, as evidenced by increased VEGF-A expression in the small intestine epithelium (p < 0.05). The addition of NAG and PTC to LP diets can improve the health and growth of weaned piglets, suggesting a potential strategy for managing the challenges associated with LP diets. This research provides valuable insights for the livestock industry, indicating that the use of natural additives like NAG and PTC can help maintain piglet health and growth while reducing the environmental impact of high-protein diets. Further research is needed to optimize these strategies for widespread application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
N-Aminoglutamate, a potent enhancer of glutamate synthase, is recognized for its ability to augment the enzyme's activity within the gastrointestinal tract, thereby facilitating the synthesis of glutamate [1, 2]. Glutamate, a crucial antioxidant, plays a pivotal role in the intestinal antioxidant defense system, counteracting oxidative stress that can induce intestinal mucosal injury [3]. As such, the incorporation of N-aminoglutamate into piglet diets is posited to fortify the intestinal antioxidant defenses and maintain intestinal integrity [4]. Pogostemon cablin, a traditional Chinese medicinal herb, is celebrated for its anti-inflammatory and antimicrobial attributes. Its extract, abundant in volatile oils and a variety of bioactive constituents, has demonstrated the capacity to quell intestinal inflammation and combat bacterial infections in piglets [5], thus mitigating inflammation and safeguarding the intestinal mucosa [6, 7].
The formulation of low-protein diets for piglets aims to alleviate the intestinal workload, a critical factor for the efficient assimilation of nutrients and the overall health and development of the animals [8]. The livestock sector grapples with challenges such as scarce protein feed resources, suboptimal nitrogen utilization efficiency, and environmental impacts. Decreasing the crude protein content in piglet diets has been proven to alleviate the protein load and reduce nitrogen excretion [9, 10], offering benefits to both animal well-being and environmental sustainability. The pursuit of augmenting farm animal growth to satisfy the market's demand for premium meat is a top priority for livestock producers [11], and enhancing meat production efficiency is of utmost importance. Post-weaning diarrhea, a prevalent health challenge in piglets, is frequently associated with the fermentation of unabsorbed proteins and amino acids, rendering the reduction of dietary protein content a strategic maneuver to bolster piglet health. Farm animal species play a vital role in meeting the global demand for meat, and they are continually evolving genetically to meet these demands. Improving the efficiency of meat production is crucial [12]. Additionally, the fermentation of unabsorbed protein and amino acids is a significant factor in post-weaning diarrhea is a common health issue in piglets [13, 14].Therefore, reducing the dietary protein content is a crucial strategy to decrease the incidence of diarrhea and improve piglet health [15].
Previous studies have assessed the individual effects of low-protein diets [16, 17] and the separate impacts of N-aminoglutamate [18] and Pogostemon cablin on piglets. However, the synergistic effects of integrating these two components with a low-protein diet have not been comprehensively explored. It is conjectured that piglets nourished with a diet enriched with both glutamate and Pogostemon cablin may display enhanced intestinal robustness, smoothing the weaning transition and diminishing stress. This investigation is a component of a more extensive research endeavor examining the combined influences of N-aminoglutamate and Pogostemon cablin extract on the intestinal health of piglets, encompassing antioxidant, barrier, and anti-inflammatory functions. The potential of these combined effects to facilitate a successful weaning transition in piglets remains uncharted territory.
2 Materials and methods
The research was undertaken at the Dacheng Animal Husbandry Commercial Farm, situated in Liaoning Province, China, with a pronounced commitment to the ethical welfare of the animals involved. The study's protocol underwent rigorous scrutiny and received approval from the Animal Protection and Use Committee at Liaoning Technical College of Agriculture, guaranteeing compliance with the pinnacle of ethical practices. The methodologies employed adhered to the directives established by the Chinese Animal Protection Committee and were meticulously executed in accordance with the ARRIVE (Animal Research: Reporting of Experimental Procedures In Vivo) guidelines. This adherence ensured that the study's procedures were meticulously designed and implemented, fulfilling all pertinent legal and regulatory stipulations.
The approval code for the study is LDSW-SOR-AAP-05–023(02).
2.1 Piglet management
One hundred twenty healthy, 28-day-old weaned piglets of the Lanximin breed, equally divided by sex, were selected for the study with an average initial weight of 7.53 ± 0.7 kg. They were randomly distributed into five distinct dietary groups: a control group with a 20.4% protein diet (CON); a low-protein group (LP) with 16.5% protein; a group receiving the LP diet supplemented with 0.2% N-acetylglutamate (LP + NAG); a group with the LP diet supplemented with 0.2% patchouli (LP + PTC); and a group receiving both supplements (LP + NAG + PTC). Each treatment comprised of six replicates with each of four piglets (2 males, 2 females). The study duration was four weeks, during which the piglets were gradually introduced to the experimental diets over a three-day period, with full implementation of the diets by the fourth day. The diet compositions, detailed in Table 1, were designed to meet the nutritional guidelines for weaned piglets as per the NRC (2012) recommendations. N-Acetylglutamine and patchouli, both with a purity of ≥ 98%, were sourced from Longyan Xin'ao Biotechnology Co., Ltd. in China. N-Acetylglutamine, with the chemical composition C7H12N2O5, is synthesized from glutamic acid and an N-acetylating agent, while patchouli is rich in volatile oils, flavonoids, and phenylpropanoids, with volatile oil being a key active component, including patchouli alcohol, ketone, and ether. The piglets were fed at regular intervals of 8:30 AM, 12:30 PM, and 5:30 PM, ensuring continuous access to food and water. The environmental conditions were meticulously controlled using an automated system, maintaining a stable temperature between 24 to 28 °C throughout the study.
2.2 Records and sample collection
Throughout the duration of the research, the body weights of the piglets were documented at 8:00 a.m. on the inaugural and penultimate days, complemented by a daily assessment of their dietary consumption. The growth metrics were assessed by determining the average daily gain (ADG), average daily feed intake (ADFI), and the weight gain to feed ratio (G:F) over the twenty-eight-day study period. Diarrheal symptoms were monitored daily, with their severity evaluated using a fecal consistency score that spanned from 0 (normal, solid feces) to 3 (severe, watery feces). The prevalence and intensity of diarrhea were quantified by calculating the proportion of affected piglets and the mean diarrhea score throughout the study.
On the concluding day, a group of 6 piglets with a male to female ratio of 1:1 were selected randomly from each treatment cohort and euthanized. Blood samples were procured via jugular vein puncture into heparinized tubes, with plasma separation achieved through centrifugation. Subsequently, the piglets were humanely euthanized via an intravenous injection of pentobarbital sodium (60 mg/kg), followed by the humane termination through the transection of the carotid artery and jugular vein. The small intestinal tissue was meticulously cleansed with saline, and the mucosa was excised using a surgical blade, rapidly cryopreserved in liquid nitrogen for subsequent analysis at − 80 °C. Additionally, a representative sample of approximately 100 g of the piglets' diet was secured for further analytical examination.
2.3 Chemical analysis
The research conducted a comprehensive analysis of immunoglobulins, including IgA, IgG, and IgM, to gauge the immune system's response in the piglets. Moreover, a spectrum of plasma cytokines was quantified, such as IL-6, IL-8, IL-10, IL-17, TGF-β, and IFN-γ, to provide a deeper insight into the inflammatory profile. The antioxidant status of the piglets was evaluated using a kit procured from the Nanjing Institute of Biology, China, adhering to the manufacturer's prescribed protocol. This evaluation encompassed the determination of total antioxidant capacity (T-AOC), catalase (CAT), total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px), total nitric oxide synthase (TNOS), and inducible nitric oxide synthase (iNOS), which are pivotal markers of the organism's capacity to combat oxidative stress.
2.4 Gene expression
Tissue specimens from the small intestine were processed by pulverization with liquid nitrogen, followed by RNA extraction utilizing Trizol reagent (R0016). The purity of the RNA was enhanced using the RNeasy Mini Kit from Qiagen, Germany, with the purity confirmed by an OD260/OD280 ratio within the range of 1.8 to 2.0. The RNA concentration was ascertained using a NanoDrop-ND2000 spectrophotometer, in accordance with the manufacturer's instructions. Subsequently, the high-quality RNA underwent reverse transcription with the Evo M-MLV reagent premix from Beyotime Biotechnology, China. Primers for the target genes were obtained from Accurate Biotechnology Co., Ltd., Hunan, China, and their validity was confirmed in the NCBI GenBank database (refer to Table 2).
Gene expression analysis was performed using real-time quantitative PCR (qPCR) with the SYBR Green method on an ABI 7900 sequence detection system. The PCR cycling conditions comprised an initial denaturation step at 94 °C for 30 s, followed by an annealing step at 60 °C for 30 s, and a final extension step at 72 °C for 30 s, with this cycle repeated 40 times. A melting curve analysis was conducted to ascertain the specificity and purity of the PCR products. The relative mRNA expression levels were quantified using the comparative Ct (cycle threshold) method formula (R = 2^-∆∆Ct, comparing the sample to a control), which facilitated the comparison of gene expression levels across various samples.
2.5 Statistical analysis
All gathered physiological and biochemical data were subjected to a stringent statistical analysis using SAS 8.1 software from SAS Institute, Inc., and GraphPad Prism version 8. For comparisons across multiple groups, a one-way analysis of variance (ANOVA) was employed, followed by a post hoc Tukey test to pinpoint specific group-wise differences. To ascertain the significance of differences between individual group pairs, standard t-tests or Holm-Sidak multiple t-tests were applied, with corrections for multiple comparisons. The Shapiro–Wilk test was implemented to assess the normality of the data distribution. The results were presented as the mean value accompanied by the standard error of the mean (SEM). A p-value of less than 0.05 was deemed to indicate statistical significance, while p-values ranging from 0.05 to 0.10 suggested marginally significant outcomes.
3 Results
3.1 Diarrhea incidence
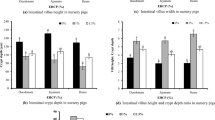
Table 3 indicates that in comparison to the control group fed the CON diet, the groups on the low-protein diet (LP), LP supplemented with N-amino-glutamate (LP + NAG), LP with patchouli extract (LP + PTC), and LP with both NAG and PTC (LP + NAG + PTC) showed a significant improvement in average daily gain (ADG) and feed efficiency (weight gain per unit of feed intake) (p < 0.05). Additionally, the average daily feed intake (ADFI) for these groups was significantly lower than that of the control group (p < 0.05). Figure 1 further illustrates that the incidence and severity of diarrhea in these groups tended to decrease when compared to the control group, although the difference did not reach statistical significance (p < 0.10).
Shows the effects of adding NAG, PTC, and the combined addition of NAG and PTC to LP feed on post-weaning piglet diarrhea incidence and diarrhea index. CON represents the basal diet; LP represents a feed with a crude protein content of 16.5%; LP + NAG represents LP feed with the addition of 0.2% N-amino-glutamate; LP + PTC represents LP feed with the addition of 0.2% patchouli extract; LP + NAG + PTC represents LP feed with the simultaneous addition of 0.2% N-amino-glutamate and 0.2% patchouli extract. a Diarrhea incidence, b Diarrhea index. Results are presented as mean ± SD
3.2 Plasma immunity, inflammatory factors and jejunal lymphocytes
Table 4 reveals that there were notable differences in plasma IgA and IgG levels among the five groups of weaned piglets (p < 0.05), while IgM levels remained unchanged (p > 0.05). Piglets in the experimental groups, which were fed diets with LP, LP + NAG, LP + PTC, and LP + NAG + PTC, exhibited a marked reduction in plasma IL-6 levels compared to those fed the CON diet (p = 0.008). Furthermore, the LP + NAG, LP + PTC, and LP + NAG + PTC groups had significantly lower IL-8 levels in their plasma than the LP group (p < 0.05), with the LP + NAG + PTC group showing the most pronounced decrease compared to the CON group (p < 0.05). Weaned piglets fed diets containing LP + NAG, LP + PTC, and LP + NAG + PTC also had increased jejunal lymphocyte counts, indicating a potentially enhanced immune response (p < 0.05).
3.3 Antioxidant capacity
As shown in Table 5, compared with piglets fed a low-protein (LP) diet, the plasma catalase (CAT) activity of piglets fed the LP diet exhibited an increasing trend (p = 0.09). Compared with the CON group and LP group, the GSH-Px activity of piglets in the LP + NAG, LP + PTC, and LP + NAG + PTC groups was significantly increased (p < 0.05). However, no significant changes were observed in the TNOS and INOS groups (p > 0.05).
3.4 mRNA expression of each related gene in the duodenum of piglets
Figure 2 illustrates that the mRNA levels of GPR2A in the duodenal mucosa of piglets from the LP + NAG, LP + PTC, and LP + NAG + PTC groups did not significantly differ from those of the CON and LP groups (p > 0.05). However, the mRNA expression of IKCA1 in the duodenal mucosa was significantly altered in the LP + NAG, LP + PTC, and LP + NAG + PTC groups compared to the CON and LP groups (p < 0.05), with the most notable changes observed in the LP + NAG + PTC group (p < 0.05). The SLC2A2 mRNA levels in the duodenal mucosa of piglets from the LP + NAG, LP + PTC, and LP + NAG + PTC groups were lower than those in the CON and LP groups, but the LP + NAG + PTC group showed a significant increase compared to the LP + NAG group (p < 0.05). The CD44 mRNA expression in the duodenal mucosa of piglets from the CON group did not significantly differ from the LP, LP + NAG, LP + PTC, and LP + NAG + PTC groups (p < 0.05), although a slight upward trend was observed (p = 0.08). In contrast, the expression of SLC12A1, VEGFA, and SLC9A3 genes in the duodenal mucosa of piglets from the LP + NAG, LP + PTC, and LP + NAG + PTC groups was significantly different compared to the CON and LP groups (p < 0.05).
The mRNA expression of GPR2A in the duodenal mucosa of weaned piglets in the LP + NAG group, LP + PTC group, and L + NAG + PTC group did not show significant changes compared to the CON group and LP group (p > 0.05). Compared to the CON group and LP group, the mRNA expression of IKCA1 in the duodenal mucosa of weaned piglets was significantly different in the LP + NAG group, LP + PTC group, and L + NAG + PTC group (p < 0.05). The content of IKCA1 in the LP + NAG + PTC group showed a more significant change than that in the other four groups (p < 0.05). Compared to the CON group and LP group, the SLC2A2 content in the duodenal mucosa of weaned piglets in the LP + NAG group, LP + PTC group, and L + NAG + PTC group was decreased. However, the content in the L + NAG + PTC group was significantly higher than that in the LP + NAG group (p < 0.05). Compared to the LP group, the LP + NAG group, the LP + PTC group, and the L + NAG + PTC group, there was no significant difference in the expression of CD44 in the duodenal mucosa of weaned piglets in the CON group (p < 0.050). However, there was an upward trend (p = 0.08). Compared to the CON group and LP group, the expression levels of the SLC12A1, VEGFA, and SLC9A3 genes in the duodenal mucosa of weaned piglets in the LP + NAG group, LP + PTC group, and L + NAG + PTC group were significantly different (p < 0.05)
4 Discussion
In our research, we observed that lowering the dietary crude protein content and supplementing it with N-acetylglutamate (NAG), patchouli extract (PTC), or a combination of both did not counteract the growth performance[19] decline typically associated with low-protein diets. This outcome is in line with previous studies, suggesting that a reduction in dietary protein by more than 3% cannot be compensated for without substantial amino acid supplementation, which is essential for the growth and development of piglets. It is imperative to ensure a balanced diet that includes both essential and non-essential amino acids to optimize protein utilization [20]. The immature intestinal development of weaned piglets makes them particularly susceptible to protein fluctuations, and the crude protein levels in our study were insufficient to support their growth.
Despite the inclusion of NAG, PTC, and their combined form, the negative impact of low protein on growth performance persisted. The reduction in protein content may have been too significant for the piglets' growth requirements to be met [21]. However, the addition of these supplements did show a positive trend in reducing the incidence and severity of diarrhea in weaned piglets, which is consistent with previous research [22]. The combined use of NAG and PTC further decreased diarrhea, positively affecting the gastrointestinal health of the piglets.
Our data indicated that the low-protein (LP) diet group tended to have lower IL-6 levels compared to the control group, and the addition of NAG and PTC, either separately or in combination, did not further alter this trend. Previous studies have demonstrated that supplementing the low-protein diet with NAG and PTC can reduce IL-6 levels [23]. In our study, the combined use of NAG and PTC reduced IL-6 levels achieved a more significant reduction compared to using either supplement alone.
The inclusion of NAG, PTC, and NAG + PTC significantly reduced IL-8 levels, particularly the combined addition of NAG + PTC, which significantly alleviated the inflammatory response. This is in agreement with previous studies that found a low-protein diet increased serum IL-8 levels in pigs, possibly due to an imbalance of amino acids [24]. Oral administration of sodium butyrate has been shown to decrease IL-8 expression in the ileum of weaned piglets [25], and both NAG and patchouli have been demonstrated to reduce IL-8 secretion by regulating homeostasis [26, 27].
Both NAG and PTC have been shown to promote the production of IL-10, an anti-inflammatory factor [28]. The promotional effect of the LP diet on IL-10 may become more evident with a longer study duration. The inclusion of NAG and PTC in low-protein diets tends to decrease IFN-γ levels, especially when used together, which aligns with other research findings. Overall, the addition of NAG, PTC, and NAG + PTC to the low-protein diet can reduce the inflammatory response, with the combination of NAG + PTC showing a particularly significant anti-inflammatory effect.
Weaning stress is a primary factor in inducing oxidative stress in the intestinal tract and blood of piglets. To combat this, antioxidant systems, including superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px), come into play to mitigate free radical production and prevent oxidative damage. Our results showed that the LP diet and its supplemented versions did not affect the levels of T-AOC, T-SOD, TNOS, and iNOS in piglet plasma. However, the LP diet seemed to upregulated CAT levels, with a noticeable recovery trend after the addition of NAG and PTC, especially in the LP + NAG + PTC group. PTC significantly increased GSH-Px levels when added to the LP diet, suggesting that PTC can enhance the antioxidant capacity of piglets, particularly elevating GSH-Px levels in the blood. NAG can increase the levels of CAT and T-SOD [29].
High-protein diets can increase the release of pro-inflammatory factors and up-regulate the expression of SLC2A2 in the intestinal tract of weaned piglets, leading to increased glucose transport [30]. This may be due to the decrease in protein and energy intake, prompting an increase in SLC2A2 expression to support the piglets' daily growth activities. However, there was no significant change in GPRC2A and CD44 expression. CD44 expression showed an upward trend, while GPRC2A expression remained relatively unchanged. This could be due to GPRC2A's role in G protein activation and its association with cell proliferation, differentiation, and apoptosis. The lack of change in GPRC2A expression might be attributed to the reduced protein content compared to normal levels. However, the intestinal mucosa damage was not significant enough to alter GPRC2A mRNA expression. Meanwhile, the increase in CD44 levels may be due to the combined effects of NAG and PTC, which aim to enhance the intestinal barrier function and improve intestinal resistance.
Compared to the control group, the gene expression levels of SLC12A1, VEGF-A, and SLC9A3 in the small intestine of the LP, LP + NAG, LP + PTC, and LP + NAG + PTC groups were significantly different and increased. A low-protein diet may promote increased intestinal absorption and ion transport, improving the ion exchange capacity and intestinal health of weaned piglets, enhancing their ability to absorb nutrients. Additionally, the increase in VEGF-A indicates the formation of vascular endothelial cells and blood vessels, enhancing material exchange capacity and indirectly improving the intestinal barrier function.
In conclusion, the addition of NAG, PTC, and the combination of NAG + PTC to a low-protein diet can enhance the anti-diarrheal and anti-inflammatory capabilities of weaned piglets, as well as improve their overall health, nutrient absorption, and intestinal barrier function. This study provides valuable insights into the dietary management of weaned piglets, particularly in the context of protein reduction and the use of natural additives to mitigate potential negative effects on growth and health. Further research is needed to optimize the balance of amino acids and the use of natural additives to ensure the best outcomes for piglet health and performance.
In addition, in the field experiment, the incomplete absorption of piglet nutrition may be caused by various objective influences and individual differences, such as differences in intestinal absorption function among different individuals or different moods among individuals at the current experimental stage. Further in-depth research is needed to address this issue.
Data availability
Data is included in the article.
References
Horak J, Lämmerhofer M. Racemization without deamidation: effect of racemization conditions on 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate tagged amino acids. J Chromatogr A. 2019;1604: 460492. https://doi.org/10.1016/j.chroma.2019.460492.
Eelen G, Dubois C, Cantelmo AR, Goveia J, Brüning U, DeRan M, et al. Role of glutamine synthetase in angiogenesis beyond glutamine synthesis. Nature. 2018;561(7721):63–9. https://doi.org/10.1038/s41586-018-0466-7.
Brosnan JT, Brosnan ME. Glutamate: a truly functional amino acid. Amino Acids. 2013;45(3):413–8. https://doi.org/10.1007/s00726-012-1280-4.
Gu X, Li Z, Wang J, Chen J, Jiang Q, Liu N, et al. Fermented cottonseed meal as a partial replacement for soybean meal could improve the growth performance, immunity and antioxidant properties, and nutrient digestibility by altering the gut microbiota profile of weaned piglets. Front Microbiol. 2021;12: 734389. https://doi.org/10.3389/fmicb.2021.734389.
Thakur A. Therapeutic potential of Pogostemon cablin herb: a comprehensive review. Pharm Patent Anal. 2022;11(6):213–24. https://doi.org/10.4155/ppa-2022-0021.
Khairan K, Hasan M, Idroes R, Diah M. Fabrication and evaluation of polyvinyl alcohol/corn starch/patchouli oil hydrogel films loaded with silver nanoparticles biosynthesized in Pogostemon cablin Benth Leaves’ extract. Molecules (Basel, Switzerland). 2023. https://doi.org/10.3390/molecules28052020.
Fatima S, Farzeen I, Ashraf A, Aslam B, Ijaz MU, Hayat S, et al. A comprehensive review on pharmacological activities of pachypodol: a bioactive compound of an aromatic medicinal plant Pogostemon Cablin Benth. Molecules (Basel, Switzerland). 2023. https://doi.org/10.3390/molecules28083469.
Ishida A, Ashihara A, Nakashima K, Katsumata M. Effects of low-protein diet and feed restriction on mRNA expression of cationic amino acid transporters in porcine skeletal muscles. Anim Sci J Nihon chikusan Gakkaiho. 2023;94(1): e13861. https://doi.org/10.1111/asj.13861.
Chrystal PV, Greenhalgh S, Selle PH, Liu SY. Facilitating the acceptance of tangibly reduced-crude protein diets for chicken-meat production. Anim Nutr (Zhongguo xu mu shou yi xue hui). 2020;6(3):247–57. https://doi.org/10.1016/j.aninu.2020.06.001.
Zhang YN, Wang S, Deng YZ, Huang XB, Li KC, Chen W, et al. The application of reduced dietary crude protein levels supplemented with additional amino acids in laying ducks. Poult Sci. 2021;100(4): 100983. https://doi.org/10.1016/j.psj.2021.01.006.
Faccin JEG, Tokach MD, Goodband RD, DeRouchey JM, Woodworth JC, Gebhardt JT. Gilt development to improve offspring performance and survivability. J Anim Sci. 2022. https://doi.org/10.1093/jas/skac128.
Soares R, Franco C, Pires E, Ventosa M, Palhinhas R, Koci K, et al. Mass spectrometry and animal science: protein identification strategies and particularities of farm animal species. J Proteomics. 2012;75(14):4190–206. https://doi.org/10.1016/j.jprot.2012.04.009.
Eriksen E, Kudirkiene E, Christensen AE, Agerlin MV, Weber NR, Nødtvedt A, et al. Post-weaning diarrhea in pigs weaned without medicinal zinc: risk factors, pathogen dynamics, and association to growth rate. Porcine Health Manag. 2021;7(1):54. https://doi.org/10.1186/s40813-021-00232-z.
Kårlund A, Gómez-Gallego C, Turpeinen AM, Palo-Oja OM, El-Nezami H, Kolehmainen M. Protein supplements and their relation with nutrition, microbiota composition and health: is more protein always better for sportspeople? Nutrients. 2019. https://doi.org/10.3390/nu11040829.
Zhang XX, Li YX, Tang ZR, Sun WZ, Wu LT, An R, et al. Reducing protein content in the diet of growing goats: implications for nitrogen balance, intestinal nutrient digestion and absorption, and rumen microbiota. Anim Int J Anim Biosci. 2020;14(10):2063–73. https://doi.org/10.1017/s1751731120000890.
Li W, Lan T, Ding Q, Ren Z, Tang Z, Tang Q, et al. Effect of low protein diets supplemented with sodium butyrate, medium-chain fatty acids, or n-3 polyunsaturated fatty acids on the growth performance, immune function, and microbiome of weaned piglets. Int J Mol Sci. 2023. https://doi.org/10.3390/ijms242417592.
Wen ZS, Tang Z, Ma L, Zhu TL, Wang YM, Xiang XW, et al. Protective effect of low molecular weight seleno-aminopolysaccharide on the intestinal mucosal oxidative damage. Mar Drugs. 2019. https://doi.org/10.3390/md17010064.
Xu QQ, Su ZR, Yang W, Zhong M, Xian YF, Lin ZX. Patchouli alcohol attenuates the cognitive deficits in a transgenic mouse model of Alzheimer’s disease via modulating neuropathology and gut microbiota through suppressing C/EBPβ/AEP pathway. J Neuroinflamm. 2023;20(1):19. https://doi.org/10.1186/s12974-023-02704-1.
Anastasaki C, Longman D, Capper A, Patton EE, Cáceres JF. Dhx34 and Nbas function in the NMD pathway and are required for embryonic development in zebrafish. Nucleic Acids Res. 2011;39(9):3686–94. https://doi.org/10.1093/nar/gkq1319.
Reyes CM, Cornelis MC. Caffeine in the diet: country-level consumption and guidelines. Nutrients. 2018. https://doi.org/10.3390/nu10111772.
Pencharz PB, Ball RO. Amino acid needs for early growth and development. J Nutr. 2004;134(6 Suppl):1566s-s1568. https://doi.org/10.1093/jn/134.6.1566S.
Peng P, Deng D, Chen S, Li C, Luo J, Romeo A, et al. The effects of dietary porous zinc oxide supplementation on growth performance, inflammatory cytokines and tight junction’s gene expression in early-weaned piglets. J Nutr Sci Vitaminol. 2020;66(4):311–8. https://doi.org/10.3177/jnsv.66.311.
Riley LK, Rupert J, Boucher O. Nutrition in toddlers. Am Fam Physician. 2018;98(4):227–33.
Chen Y, Xie Y, Zhong R, Han H, Liu L, Chen L, et al. Effects of graded levels of xylo-oligosaccharides on growth performance, serum parameters, intestinal morphology, and intestinal barrier function in weaned piglets. J Anim Sci. 2021. https://doi.org/10.1093/jas/skab183.
Sun Q, Ji YC, Wang ZL, She X, He Y, Ai Q, et al. Sodium butyrate alleviates intestinal inflammation in mice with necrotizing enterocolitis. Mediat Inflamm. 2021;2021:6259381. https://doi.org/10.1155/2021/6259381.
Yang HM, Zhuo JY, Sun CY, Nie J, Yuan J, Liu YL, et al. Pogostone attenuates TNF-α-induced injury in A549 cells via inhibiting NF-κB and activating Nrf2 pathways. Int Immunopharmacol. 2018;62:15–22. https://doi.org/10.1016/j.intimp.2018.06.029.
Henrick BM, Nag K, Yao XD, Drannik AG, Aldrovandi GM, Rosenthal KL. Milk matters: soluble Toll-like receptor 2 (sTLR2) in breast milk significantly inhibits HIV-1 infection and inflammation. PLoS ONE. 2012;7(7): e40138. https://doi.org/10.1371/journal.pone.0040138.
Whaley-Connell AT, Habibi J, Nistala R, DeMarco VG, Pulakat L, Hayden MR, et al. Mineralocorticoid receptor-dependent proximal tubule injury is mediated by a redox-sensitive mTOR/S6K1 pathway. Am J Nephrol. 2012;35(1):90–100. https://doi.org/10.1159/000335079.
Sun DQ, Li AW, Li J, Li DG, Li YX, Hao F, et al. Changes of lipid peroxidation in carbon disulfide-treated rat nerve tissues and serum. Chem Biol Interact. 2009;179(2–3):110–7. https://doi.org/10.1016/j.cbi.2008.11.014.
Nolde M, Alayash Z, Reckelkamm SL, Kocher T, Ehmke B, Holtfreter B, et al. Downregulation of interleukin 6 signaling might reduce the risk of periodontitis: a drug target Mendelian randomization study. Front Immunol. 2023;14:1160148. https://doi.org/10.3389/fimmu.2023.1160148.
Funding
This article has no funding support.
Author information
Authors and Affiliations
Contributions
L,SC:Conceptualization, Methodology, Software, Investigation, Formal Analysis, Writing - Original Draft;L,MZ: Data Curation, Writing - Original Draft;S,GB: Visualization, Investigation;Z,R:Resources, Supervision;D,Y:Software, ValidationZ,Y: Visualization, Writing - Review & EditingL,MX ,Q,HMand L,WX: Conceptualization, Funding Acquisition, Resources, Supervision, Writing - Review & Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, S., Shao, G., Li, M. et al. Effect of the supplementation of N-acetylglutamate and patchouli extract on piglets fed on a low protein diet. Discov Anim 1, 12 (2024). https://doi.org/10.1007/s44338-024-00011-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44338-024-00011-4