Abstract
This review presents a comprehensive review of the literature regarding the effects of non-steroidal anti-inflammatory drugs (NSAIDs) on bone healing outcomes in animal models. A thorough search was conducted using PubMed, Web of Science, and Google Scholar, resulting in the inclusion of 27 papers that met the predetermined criteria. The majority of the studies utilized rodent and rabbit models, with limited research conducted on dogs and no studies on other animal species. The findings from the included studies presented conflicting evidence on the impact of NSAIDs on bone healing, with some reporting negative effects such as delayed healing and impaired bone formation. Interestingly, no consistent relationship was found between the type, potency, dosage, or timing of NSAID administration and the observed effects. Various methodological limitations were identified in the reviewed studies, including small sample sizes, lack of diverse animal populations, and inadequate statistical analyses. It is important to address these limitations and conduct further research, particularly involving companion and farm animals, to improve the clinical relevance of the findings. Caution should be exercised when using NSAIDs in the management of bone fractures, and a more comprehensive understanding of their effects on bone healing is needed to optimize treatment strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Bone healing is a highly intricate process aimed at repairing fractured bones without the formation of scar tissue. It involves a series of regenerative changes in the affected bone segment, ultimately restoring its pre-fracture integrity and structural stability [1]. Two main forms of bone healing, primary and secondary, are distinguished based on the distance between the fractured bone fragments. Primary bone healing occurs when the cortex is restored without the formation of a callus, typically achieved through proper fracture reduction and immobilization. On the other hand, secondary bone healing involves the formation of a callus and subsequent remodeling [2]. Immediately following a fracture, the disruption of blood supply, hypoxia, and the presence of a hematoma trigger secondary fracture healing processes.
1.1 Primary bone healing
Primary healing is a process that enables the rapid reconstruction of bone structure, particularly through the restoration of the Haversian and anatomical lamellar systems. Primary healing takes place when the bone fragments are precisely aligned, brought together, and securely stabilized through compression, preventing any movement at the site of the fracture. When these conditions are met, the bone has the potential to undergo healing directly through the remodeling of lamellar bone and Haversian canals [3]. The bone on either side of the cortex needs to reconnect for mechanical and physical integrity to be restored. Cutting cones develop at the edges of the osteons that are nearest to the location of the fracture. These cones traverse the fracture boundary and create elongated voids (by osteoclasts), which are subsequently filled with bone matrix by osteoblasts. This process leads to the formation of solid bone connections and the revival of Haversian systems, enabling the re-establishment of blood supply. Ultimately, osteons mature and transform into lamellar bone, facilitating the mending of the fracture site without the occurrence of callus formation or inflammation [2, 3]. Surgical interventions play a role in bypassing the initial stages of secondary healing, promoting primary bone healing. The implementation of rigid fracture fixation creates a low interfragmentary tension environment, which triggers the formation of lamellar bone and subsequent Haversian remodeling [2].
1.2 Secondary bone healing
The process of fracture healing can be categorized into three main phases: inflammation, regeneration, and remodeling. These phases occur sequentially but partially also overlap [2, 3]. The initial stage, inflammation, involves the formation of a callus, which lasts until the completion of a soft fibrous callus [2,3,4]. Trauma-induced hematomas play a crucial role in fracture repair by filling the gap between the fractured ends and the underlying soft tissues. Fibroblasts, derived from mesenchymal stem cells, attach to collagens and form a collagen matrix, while osteoblasts, also derived from mesenchymal stem cells, initiate the formation of spongy bone at fracture [2, 3]. Granulation tissue forms between the fracture fragments, and precursor cells migrate to the hematoma where they differentiate into macrophages and osteoblasts. These cells are responsible for phagocytosis, which involves the engulfment of debris and pathogens, and fibroplasia, the formation of fibrous tissue essential for callus formation [3]. Inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), prostaglandin E2, granulocyte–macrophage colony-stimulating factor, monocyte colony-stimulating factor, and interleukin-1 (IL-1), are released at the fracture site by macrophages and fibroblasts, promoting leukocyte migration and fibroblast collagen formation. Venous endothelial growth factor (VEGF), produced by various cell types including endothelial cells and macrophages, stimulates vascular endothelium [3]. Osteoclastic activity by hematopoietic stem cells (specifically from cells of the monocyte-macrophage lineage) removes dead bone tissue, which is then followed by osteogenesis. [2, 3]. The repair phase follows the inflammatory phase, with osteoblasts and chondroblasts, derived from mesenchymal stem cells, migrating to the blood clot and initiating fibroplasia to form collagen-rich fibrous tissue. This tissue serves as the framework for the subsequent formation of a soft fibrous callus, which stabilizes the fractured bone. Osteoblasts and chondroblasts differentiate into mature osteoids and chondrocytes and form a callus between the fractured ends. Despite its hardening, the callus does not possess the full weight-bearing capacity. As callus forms, stability between the fracture ends increases, and blood vessels in the bone medulla initiate the remodeling phase [2]. Bone cellular units, consisting of osteoclasts, osteoblasts, and osteocytes, remodel the bone. Different cell types make up bone multicellular units; some are focused on resorbing old bone, while others are tasked with creating new bone to replace the lost. Each bone cellular unit is made up of a variety of morphologically and functionally distinct cell types, namely osteocytes, osteoblasts, and osteoclasts, which work together on the bone remodeling compartment to replace the worn-out bone with new bone. Osteoblasts from the periosteum and endosteum contribute to osteoid formation, which eventually becomes the bone matrix. Osteoclasts play a role in the remodeling phase by resorbing new bone tissue in the callus until it reaches a size comparable to its natural formation. This phase also involves the formation of lamellar bone structure (osteoid) in the Haversian canals, signifying the completion of bone healing [2, 3].
1.3 Non-steroidal anti-inflammatory drugs (NSAID)
Medications in the class of nonsteroidal anti-inflammatory drugs (NSAIDs) can suppress the four cardinal signs of inflammation: rubor, tumor, dolor, and calor. The use of NSAIDs became increasingly widespread during the 1990s as more knowledge was gained regarding their dosing and administration [5]. Initially used for treating osteoarthritis, the discovery of carprofen and deracoxib expanded their applications over time [6]. NSAIDs are widely utilized due to their therapeutic effects on the body, including pain relief, antipyretic properties, and anti-inflammatory actions, making them effective in managing both acute and chronic pain. These drugs can reach target tissues continuously by binding to plasma proteins, with effects typically occurring within 30 to 60 min and lasting for more than 24 h [5, 6]. NSAIDs exhibit chemical diversity and most of them function by inhibiting the cyclooxygenase (COX) enzyme. COX enzymes exist in two forms: COX-1 and COX-2. COX-1 is present in various tissues such as blood vessels, interstitial cells, smooth muscle cells, platelets, and mesothelial cells throughout the body and is involved in intercellular communication, regulation of prostaglandin synthesis, and protection of the gastric mucosa [5,6,7]. Many of the side effects associated with NSAIDs can be attributed to the inhibition of this enzyme [5]. In contrast, the COX-2 enzyme is primarily found in inflammatory tissue, possesses hemostatic properties [8], and contributes to the production of prostanoids, which trigger the inflammatory response [5,6,7].
The majority of commercially available NSAIDs inhibit both COX-1 and COX-2 enzymes rather than selectively targeting either one. Studies indicate that NSAIDs specifically targeting the COX-2 enzyme exert the most pronounced analgesic and anti-inflammatory effects compared to those mediated by COX-1 [5]. Inhibition of the COX-2 enzyme signifies the potency of NSAIDs in producing analgesic and anti-inflammatory effects [5]. The nonsteroidal anti-inflammatory drugs act by selectively inhibiting both COX-1 and COX-2 enzymes to suppress prostaglandin synthesis. Dogs and cats are reported to be more susceptible to adverse effects from NSAIDs compared to other species [5, 9].
During fracture healing, prostaglandins play a crucial role as lipid mediators synthesized from arachidonic acid by cyclooxygenase enzymes (COX-1 and COX-2) [5]. These enzymes regulate inflammation and are vital for prostaglandin production, which stimulates both bone formation and resorption [7, 8]. Prostaglandins enhance chondrocyte growth, differentiation, and osteogenic cell proliferation, essential for healing [4, 5]. Prostaglandin E2 (PGE2) influences bone metabolism by promoting bone formation in response to mechanical stress and fractures while contributing to bone loss in inflammatory conditions [5,6,7]. Its effects depend on binding to specific E-prostanoid receptors, either stimulating bone formation or promoting resorption [4,5,6,7]. NSAIDs inhibit COX activity, reducing prostaglandin synthesis, which can impede normal bone turnover and fracture healing.
This study aims to comprehensively review and consolidate existing data regarding the impact of NSAID administration on outcome measurements of bone healing in various animal models following bone fracture surgery.
2 Methods
We conducted a comprehensive search of available literature on PubMed, Web of Science, and Google Scholar, using keywords such as “osteotomy”, “fracture”, “bone healing”, and various animal species including “cat”, “mouse”, “mice”, “rat”, “rabbit”, and “dog”, as well as generic terms like “NSAID”. We searched three databases, with the search parameters not restricted by a specific start date, up to January 2, 2023, aiming to identify articles focused on bone healing in animal models with administered NSAIDs following fractures. Articles were then evaluated for inclusion based on predetermined criteria, which involved (1) an interventional design that included NSAIDs treatment following bone fracture, and (2) outcome measures related to bone healing, including biomechanical characteristics, μ-CT scan measures, biochemical analysis, radiographic bone assessment, and/or histomorphometric and histological-based evaluations. Articles were excluded if they met any of the following criteria: not being an original article (such as a review, case report, or letter), use of a bone graft or other materials, reporting outcomes unrelated to biomechanics or histomorphometry, not involving NSAID use, not involving experimental animal studies (in vitro studies), conference abstracts, or if they were duplicate studies.
3 Results
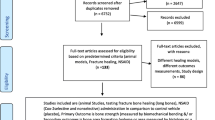
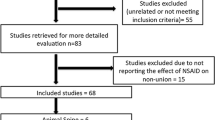
A total of 1613 studies were screened initially, and after a comprehensive evaluation of the full texts, 27 papers met the inclusion criteria and were included in this review. The selection process for these papers is illustrated in Fig. 1, providing a clear visualization of the screening and inclusion steps. The distribution of the included studies is depicted in Fig. 2 and 3, showcasing the classification based on the type of NSAID (COX, COX-2 selective), the animal subjects utilized, and the corresponding findings. It is noteworthy that a significant number of studies conducted over the past two decades have utilized experimental animal models of fractures, with rodents accounting for 17 studies and rabbits accounting for 8 studies, indicating their predominant use in this field of research.
Distribution of studies using non-selective COX NSAIDs in the treatment group. The different colors in the bars represent different species (rabbits, rats, mice, etc.). LD Low-dose treatment, LT Long-term treatment. Not suitable represents studies that used multiple NSAID treatments and have contradictory results with each other
The majority of the published articles in this review examined laboratory animals, with a focus on mice (3 studies), rats (14 studies), and rabbits (8 studies), while only two articles specifically investigated dogs. The identified research encompasses a wide range of outcomes, and it is important to note that some of the findings presented in Table 1 are conflicting. Several studies indicate that NSAIDs have adverse effects on bone physiology, leading to delayed bone healing, impaired callus formation, and compromised mechanical properties of bones. Notably, some authors have conducted comparisons between the effect of NSAIDs on fracture healing and that of other drugs [11, 12].
Several studies have presented conflicting evidence regarding the impact of NSAIDs on fracture healing. Interestingly, even when utilizing identical animal fracture models, the same drugs, and the same doses, these studies have yielded inconsistent results. Upon closer examination, we did not find a consistent correlation between the class or potency of NSAIDs, their ability to inhibit COX-1 or COX-2 enzymes, the dosage, or the timing of administration with respect to their effect on bone healing in published studies. While some researchers suggest that short-term use of NSAIDs after a fracture is safe, others argue that it is only safe if initiated several weeks after the fracture, as indicated in Table 1. However, one potential correlation that emerged from the analysis is the species of the animals used in the studies. The majority of the animal models employed were rodents and rabbits, with only two studies involving dogs and no inclusion of other species such as farm or companion animals (Table 1). Numerous research studies have provided evidence demonstrating that various traditional NSAIDs, including aspirin, indomethacin, ibuprofen, meloxicam, naproxen, and ketoprofen, as well as COX-2 selective coxib medications, hinder bone healing in diverse animal models, as summarized in Table 1.
4 Discussion
A comprehensive literature search yielded 27 studies that met the inclusion criteria [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32, 34,35,36,37]. A limited number of studies reported insignificant or no effect of NSAIDs on fracture healing outcomes [12, 26,27,28,29,30,31]. However, these studies have notable limitations, such as evaluating only a single time point and failing to quantify well-defined bone healing outcomes encompassing both mechanical and histomorphometric analyses [13,14,15, 18, 19, 24, 26, 27, 31, 32], and most of them lacked robust statistical analyses. A 2019 review by Huss et al. emphasized the need for a more rigorous and evidence-based approach to assessing the risks and benefits of NSAID administration in orthopedic treatments [38].
The laboratory animal data and its clinical translation may be prone to potential methodological issues, requiring careful discussion to avoid misinterpretation. In our review, we included detailed data to explore factors that could modify the effect of NSAIDs on bone healing outcomes. These detailed data factors encompassed animal species, sex, age, weight, bone type, drug, doses, duration, way of administration, and outcome measurements (Table 1).
To accurately assess the effect of NSAIDs on bone healing, it is crucial to consider potential pharmacokinetic variations between different animal species, as well as between sexes and age groups within a species [33]. Notably, the reviewed studies did not incorporate both male and female animals, despite our findings indicating differences in work-to-failure biomechanical outcomes between the sexes, which highlights the importance of such considerations in future studies (Table 1).
Furthermore, the studies included in our analysis employed various opioids (buprenorphine, butorphanol, morphine), and antibiotics (enrofloxacin, cefazolin) alongside the investigated drugs, administered at varying times and doses before and after the interventions, which was recognized as a limitation in those studies [12, 17, 21, 23, 26, 34]. Hence, it is crucial to consider factors like drug absorption and administration routes within all aspects of pharmacology when designing and interpreting experimental studies. The treatment durations, routes of administration, and doses of the used NSAIDs also exhibited variability across the reviewed studies (Table 1).
Additionally, our findings should be interpreted cautiously due to several limitations in the included studies. Most animal models used in the review were healthy, which may limit their representativeness of real-world pathological conditions (Table 1). Moreover, except for four studies [11, 12, 20, 29], sample size calculations were not performed in the remaining studies, potentially impacting the reliability and generalizability of the results. None of the reviewed studies provided robust statistics for data analysis, such as using a wide range of probability distributions or enhancing the precision and accuracy of measurement devices and procedures. Moreover, they did not report the choice of using a one-tailed/two-tailed test for used t-tests and z-tests in statistical analyses. This lack of methodological rigor is considered a limitation, affecting the validity of the studies’ conclusions.
Limited studies are available on companion animals [36, 37], and these studies have certain limitations, such as the absence of sample size analyses and reliance on a small number of samples. These studies focused on the effects of carprofen, administered to dogs, on bone healing over short and long periods, specifically targeting the tibia. Although the results suggested a negative impact of long-term use of COX-2 selective NSAIDs on bone healing (Table 1), they contradict some studies conducted on laboratory animals, emphasizing the need for further research with larger sample sizes to achieve a comprehensive understanding.
When selecting an animal model for bone regeneration experiments, it is essential to consider skeletal characteristics, relevance to clinical scenarios, appropriate doses, and statistically supported sample sizes. Accounting for these factors enhances the relevance and translatability of experimental results to both companion animals and human patients [39].
In summary, the potential methodological issues associated with laboratory animal data and its clinical translation should be acknowledged and discussed to ensure accurate interpretation. Our review revealed the importance of considering factors such as species, sex, age, drug characteristics, aspects of pharmacology, and outcome measurements when studying the effects of NSAIDs on bone healing. The variability in drug administration and absorption should also be considered during study design and result interpretation. Additionally, the limitations observed in the included studies, including the lack of diverse animal populations and sample size calculations, highlight areas for improvement in future research. Further investigations involving companion animals are necessary to expand our understanding of NSAID effects on bone healing and to bridge the gap between animal models and human patients. By addressing these considerations, we can enhance the validity and clinical relevance of experimental findings in the field of bone healing.
The reviewed studies collectively highlight the varied impact of NSAIDs on bone healing, contingent on dosage and duration (Table 1; Figs. 2 and 3). High doses of NSAIDs, such as diclofenac and aspirin, have been linked to significant delays in bone healing [10, 24]. Long-term administration of NSAIDs like rofecoxib and carprofen consistently delayed bone healing [17, 21, 36, 37]. Conversely, short-term or lower-dose treatments with NSAIDs like flunixin meglumine and meloxicam demonstrated less detrimental effects on bone healing [14, 31]. The inhibition of COX enzymes and the reduction in prostaglandin synthesis, crucial for osteoblast and osteoclast activity, underlie these effects [1, 2, 20, 34]. Therefore, careful consideration of NSAID dosage and treatment duration is essential to balance effective pain management with minimal negative impact on bone repair processes.
The use of NSAIDs has long been established in posttraumatic pain management; however, their impact on bone healing has been a subject of concern in the literature (Table 1; Figs. 2 and 3). Animal studies investigating NSAID effects on newly formed bone have utilized varying doses and treatment durations, resulting in significant diversity and conflicting outcomes (Table 1; Fig. 2 and 3). The lack of a definitive conclusion on the mechanism of action and effect of NSAIDs on bone healing in animals raises questions about the reliability of numerous studies. Inconsistencies were observed not only across different species, doses, routes of administration, and treatment durations but also among studies examining identical parameters. The underlying factors contributing to these variations remain unclear and may stem from differences between or within species, compensatory local and systemic factors, or variations in drug pharmacokinetics between laboratory animal models and other species. Additional factors such as the achieved level of pain relief and the weight-bearing status of animals could also influence the healing process. We believe that the extent of trauma and fracture comminution observed in these experimental models plays a significant role in the discrepancies among researchers. Variations in fracture comminution, applied force, soft tissue damage, and achieved fracture stability can all contribute to the outcome.
In conclusion, high-dose and long-term NSAID use consistently delayed healing, contrasting with milder effects of short-term and lower-dose regimens. By inhibiting cyclooxygenase (COX) enzymes, NSAIDs reduce prostaglandin synthesis, which is vital for inflammation and bone healing. This inhibition can delay or impair bone healing by decreasing osteoblast activity, increasing osteoclast activity, and impairing angiogenesis. While reducing inflammation can benefit muscle tissues by alleviating pain and soreness, NSAIDs might also hinder muscle repair and regeneration by affecting protein synthesis. In blood vessels, NSAIDs can cause vasoconstriction, impair endothelial function, and reduce thromboxane synthesis, impacting vascular health and healing processes. Standardized methodologies and robust statistical approaches are crucial to clarify NSAID effects across species and optimize clinical fracture management. Further research should refine methodological standards, including rigorous sample size calculations and standardized outcome measurements, to enhance reliability and applicability. Addressing variations between laboratory animal models and companion animals can advance translational research and clinical outcomes in bone healing interventions.
Data availability
The data that support the findings of this study are available from the corresponding author (Yalcin Alper OZTURAN), upon reasonable request. No datasets were generated or analysed during the current study.
References
Marsh DR, Li G. The biology of fracture healing: optimizing outcome. Br Med Bull. 1999;55:856–89. https://doi.org/10.1258/0007142991902673.
Dejardin LM, Saunders WB. Biomechanics and fracture biology. In: Johnston S, Tobias K, editors. veterinary surgery: small animal expert consult. St. Louis: Elsevier; 2018. p. 613–49.
Remedios A. Bone and bone healing. Vet Clin North Am Small Anim Pract. 1999;29:1029–44. https://doi.org/10.1016/s0195-5616(99)50101-0.
Hulse D, Hyman B. Fracture biology and biomechanics. In: Slatter DH, editor. Textbook of small animal surgery. Philadelphia: WB Saunders; 2003. p. 1785.
Stiller CO, Hjemdahl P. Lessons from 20 years with COX-2 inhibitors: Importance of dose–response considerations and fair play in comparative trials. J Intern Med. 2022;292:557–74. https://doi.org/10.1111/joim.13505.
Lascelles BDX, Butterworth SJ. Postoperative analgesic and sedative effects of carprofen and pethidine in dogs. Vet Rec. 1994;134:187–91. https://doi.org/10.1136/vr.134.8.187.
Lees P, Landoni MF, Giraudel J, Toutain PL. Pharmacodynamics and pharmacokinetics of nonsteroidal anti-inflammatory drugs in species of veterinary interest. J Vet Pharmacol Ther. 2004;27:479–90. https://doi.org/10.1111/j.1365-2885.2004.00617.x.
Lascelles BDX, Mcfarland MJ. Guidelines for safe and effective use of NSAIDs in dogs. Vet Ther. 2005;6:1–15.
Kis B, Snipes JA, Busija DW. Acetaminophen and the COX-3 puzzle: sorting out facts, fictions, and uncertainties. J Pharmacol Exp Ther. 2005;315:1–7. https://doi.org/10.1124/jpet.105.085431.
Beck A, Krischak G, Sorg T, Augat P, Farker K, Merkel U, Kinzl L, Claes L. Influence of diclofenac (group of nonsteroidal anti-inflammatory drugs) on fracture healing. Arch Orthop Trauma Surg. 2003;123:327–32. https://doi.org/10.3390/biomimetics7040143.
Karachalios T, Boursinos L, Poultsides L, Khaldi L, Malizos KN. The effects of the short-term administration of low therapeutic doses of anti-COX-2 agents on the healing of fractures. An experimental study in rabbits. J Bone Joint Surg Br. 2007;89:1253–60. https://doi.org/10.1302/0301-620X.89B9.19050.
Bissinger O, Kreutzer K, Götz C, Hapfelmeier A, Pautke C, Vogt S, Wexel G, Wolff KD, Tischer T, Prodinger PM. A biomechanical, micro-computertomographic and histological analysis of the influence of diclofenac and prednisolone on fracture healing in vivo. BMC Musculoskelet Disord. 2016;17:383. https://doi.org/10.1186/s12891-016-1241-2.
Goodman S, Ma T, Trindade M, Ikenoue T, Matsuura I, Wong N, Fox N, Genovese M, Regula D, Smith RL. COX-2 selective NSAID decreases bone ingrowth in vivo. J Orthop Res. 2002;20:1164–9. https://doi.org/10.1016/S0736-0266(02)00079-7.
Ribeiro FV, César-Neto JB, Nociti FH, Sallum EA, Sallum AW, De Toledo S, Casati MZ. Selective cyclooxygenase-2 inhibitor may impair bone healing around titanium implants in rats. J Periodontol. 2006;77:1731–5. https://doi.org/10.1902/jop.2006.060119.
Kaygusuz MA, Turan CC, Aydin NE, Temel I, First S, Bulut T, Kuku I. The effects of G-CSF and naproxen sodium on the serum TGF-beta1 level and fracture healing in rat tibias. Life Sci. 2006;80:67–73. https://doi.org/10.1016/j.lfs.2006.08.023.
Murnaghan M, Li G, Marsh DR. Nonsteroidal anti-inflammatory drug-induced fracture nonunion: an inhibition of angiogenesis? J Bone Joint Surg Am. 2006;88:140–7. https://doi.org/10.2106/JBJS.F.00454.
Mullis BH, Copland ST, Weinhold PS, Miclau T, Lester GE, Bos GD. Effect of COX-2 inhibitors and non-steroidal anti-inflammatory drugs on a mouse fracture model. Injury. 2006;37:827–37. https://doi.org/10.1016/j.injury.2005.12.018.
Tiseo BC, Namur GN, de Paula EJ, Junior RM, de Oliveira CR. Experimental study of the action of COX-2 selective nonsteroidal anti-inflammatory drugs and traditional anti-inflammatory drugs in bone regeneration. Clinics. 2006;61:223–30. https://doi.org/10.1590/s1807-59322006000300007.
Krischak GD, Augat P, Sorg T, Blakytny R, Kinzl L, Claes L, Beck A. Effects of diclofenac on periosteal callus maturation in osteotomy healing in an animal model. Arch Orthop Trauma Surg. 2007;127:3–9. https://doi.org/10.1007/s00402-006-0202-x.
Gerstenfeld LC, Al-Ghawas M, Alkhiary YM, Cullinane DM, Krall EA, Fitch JL, Webb EG, Thiede MA, Einhorn TA. Selective and nonselective cyclooxygenase-2 inhibitors and experimental fracture-healing. Reversibility of effects after short-term treatment. J Bone Joint Surg Am. 2007;89:114–25. https://doi.org/10.2106/JBJS.F.00495.
O’Connor JP, Capo JT, Tan V, Cottrell JA, Manigrasso MB, Bontempo N, Parsons JR. A comparison of the effects of ibuprofen and rofecoxib on rabbit fibula osteotomy healing. Acta Orthop. 2009;80:597–605. https://doi.org/10.3109/17453670903316769.
Ribeiro FV, Nociti FH, Sallum EA, Casati MZ. Effect of aluminum oxide-blasted implant surface on the bone healing around implants in rats submitted to continuous administration of selective cyclooxygenase-2 inhibitors. Int J Oral and Maxillofac Surg. 2009;24:226–33.
Hak DJ, Schulz KS, Khoie B, Hazelwood SJ. The effect of Cox-2 specific inhibition on direct fracture healing in the rabbit tibia. J Orthop Sci. 2011;16:93–8. https://doi.org/10.1007/s00776-010-0016-0.
Lack WD, Fredericks D, Petersen E, Donovan M, George M, Nepola J, Smucker J, Femino JE. Effect of aspirin on bone healing in a rabbit ulnar osteotomy model. J Bone Joint Surg Am. 2013;95:488–96. https://doi.org/10.2106/JBJS.L.00462.
Kidd LJ, Cowling NR, Wu AC, Kelly WL, Forwood MR. Selective and non-selective cyclooxygenase inhibitors delay stress fracture healing in the rat ulna. J Orthop Res. 2013;31:235–42. https://doi.org/10.1002/jor.22203.
Cappello T, Nuelle JA, Katsantonis N, Nauer RK, Lauing KL, Jagodzinski JE, Callaci JJ. Ketorolac administration does not delay early fracture healing in a juvenile rat model: a pilot study. J Pediatr Orthop. 2013;33:415–21. https://doi.org/10.1097/BPO.0b013e318288b46f.
Inal S, Kabay S, Cayci MK, Kuru HI, Altikat S, Akkas G, Deger A. Comparison of the effects of dexketoprofen trometamol, meloxicam, and diclofenac sodium on fibular fracture healing, kidney, and liver: an experimental rat model. Injury. 2014;45:494–500. https://doi.org/10.1016/j.injury.2013.10.002.
Sandberg O, Aspenberg P. Different effects of indomethacin on healing of shaft and metaphyseal fractures. Acta Orthop. 2015;86:243–7. https://doi.org/10.3109/17453674.2014.973328.
Janssen MP, Caron M, van Rietbergen B, Surtel DA, van Rhijn LW, Welting TJ, Emans PJ. Impairment of the chondrogenic phase of endochondral ossification in vivo by inhibition of cyclooxygenase-2. Eur Cells Mater. 2017;34:202–16. https://doi.org/10.22203/eCM.v034a13.
Sen C, Erdem M, Gunes T, Koseoglu D, Filiz NO. Effects of diclofenac and tenoxicam on distraction osteogenesis. Arch Orthop Trauma Surg. 2007;127:153–9. https://doi.org/10.1007/s00402-006-0274-7.
Elgendy M, Elsayad G, Seleim M, Abdo W, Baty RS, Elmahallawy EK, Atiba A. Flunixin meglumine enhanced bone fracture healing in rabbits associated with activation of early collagen deposition and enhancement of vascular endothelial growth factor expression. Animals. 2021;11:2834. https://doi.org/10.3390/ani11102834.
Pablos AB, Ramalho SA, König B, Furuse C, Cury PR. Effect of meloxicam and diclofenac sodium on peri-Implant bone healing in rats. J Periodontol. 2008;79:300–6. https://doi.org/10.1902/jop.2008.070301.
Miyatake S, Ichiyama H, Kondo E, Yasuda K. Randomized clinical comparisons of diclofenac concentration in the soft tissues and blood plasma between topical and oral applications. Br J Clin Pharmacol. 2009;67:125–9. https://doi.org/10.1111/j.1365-2125.2008.03333.x.
Gerstenfeld LC, Thiede M, Seibert K, Mielke C, Phippard D, Svagr B, Cullinane D, Einhorn TA. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res. 2003;21:670–5. https://doi.org/10.1016/S0736-0266(03)00003-2.
Bergenstock M, Min W, Simon AM, Sabatino C, O’Connor JP. A comparison between the effects of acetaminophen and celecoxib on bone fracture healing in rats. J Orthop Trauma. 2005;19:717–23. https://doi.org/10.1097/01.bot.0000184144.98071.5d.
Ochi H, Hara Y, Asou Y, Harada Y, Nezu Y, Yogo T, Shinomiya K, Tagawa M. Effects of long-term administration of carprofen on healing of a tibial osteotomy in dogs. Am J Vet Res. 2011;72:634–41. https://doi.org/10.2460/ajvr.72.5.634.
Gallaher HM, Butler JR, Wills RW, Priddy LB, Elder SH, Heller SM, Brinkman E, Baumgartner W. Effects of short- and long-term administration of nonsteroidal anti-inflammatory drugs on osteotomy healing in dogs. Vet Surg. 2019;48:1318–29. https://doi.org/10.1111/vsu.13282.
Huss MK, Felt SA, Pacharinsak C. Influence of pain and analgesia on orthopedic and wound-healing models in rats and mice. Comp Med. 2019;69:535–45. https://doi.org/10.30802/AALAS-CM-19-000013.
Peric M, Dumic-Cule I, Grcevic D, Matijasic M, Verbanac D, Paul R, Grgurevic L, Trkulja V, Bagi CM, Vukicevic S. The rational use of animal models in the evaluation of novel bone regenerative therapies. Bone. 2015;70:73–86. https://doi.org/10.1016/j.bone.2014.07.010.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Y.A.O. worked on the conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, visualization, and project administration and wrote the main manuscript text. I.A. worked on methodology, validation, data curation and supervision of the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
All authors declare that they have no conflicts of interest/competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ozturan, Y.A., Akin, I. Comprehensive review of the impact of NSAIDs on bone healing outcomes in animal models: conflicting evidence and methodological considerations. Discov Med 1, 20 (2024). https://doi.org/10.1007/s44337-024-00034-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44337-024-00034-6