Abstract
Background
Acanthosis nigricans (AN) presents as hyperpigmented plaques with ill-defined borders, commonly in intertriginous areas and flexures, and is associated with various factors, including obesity, insulin resistance, and malignancies. Recognizing the clinical significance of ANs relationship with metabolic syndrome and cardiovascular risk is important for early intervention and prevention.
Purpose
This study describes the metabolic pathways underlying AN, highlighting its association with hyperinsulinemia, insulin-like growth factor 1 (IGF1), leptin, and their receptors. The pathophysiology involves disruptions in insulin, IGF1, leptin, fibroblast growth factor receptors (FGFR) and epidermal growth factor receptors (EGFR), leading to keratinocyte and fibroblast proliferation.
Methods
A comprehensive literature search through PubMed was performed. Terms such as “Acanthosis nigricans”, “hyperinsulinemia”, “cardiovascular disease”, “diabetes”, “coronary artery disease”, “hyperleptinemia”, “obesity”, “leptin”, “epidermal growth factor”, “insulin resistance”, were brought into consideration. Further articles were found using source materials from included references. Articles published from 1980 to 2023 were used to encompass the broad range of research covered on the subjects.
Results
AN is connected to insulin, IGF1, leptin, and growth factor receptors and is an independent marker for metabolic disorders.
Discussion
Dermatologists may intervene by referring to primary care or by addressing underlying causes such as obesity and hyperinsulinemia, emphasizing the importance of weight loss. Various treatments—including medication, topical therapies, and laser modalities—may provide limited improvement. Recognizing AN's significance in cardiovascular and metabolic disease could ote detection and prevention of cardiovascular diseases, improving patient outcomes.
Key points
AN is an independent marker for metabolic disorders and its potential for further prompting into a metabolic and cardiovascular risk assessments or referral. Recognizing the metabolic connections in AN is important for dermatologists in intervening early and preventing cardiovascular and metabolic comorbidities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
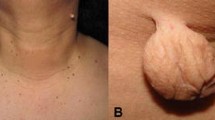
Acanthosis nigricans (AN) is characterized by thickened velvety hyperpigmented plaques. The plaques mainly appear in intertriginous areas and flexures, most commonly on the axilla, neck, and groin, but AN can present on various areas of the body, especially if associated with malignancy. AN can be seen on the elbows, the dorsal joint surfaces of the hands, face, eyelids, genitals, and the oral mucosa [1]. The disease was first described in 1890 in the International Atlas of Rare Skin Diseases and was associated with malignancy. Since that time AN has been linked to endocrinopathies and medication, but most importantly to obesity and insulin resistance.
Obesity is strongly associated with AN [2]. In adolescents, AN prevalence reaches 66% among those above 200% of their ideal body weight, with higher rates in Hispanic (5.5%) and African American (13.3%) individuals [3]. Among obese participants, AN prevalence can be over 60% [4] and as high as 74% of adults attending obesity clinics [5].
AN and metabolic syndrome are associated with hyperinsulinemia. Fasting insulin levels are approximately twofold higher than normal in individuals with AN [6]. In Native American children, AN is an independent marker for insulin resistance, with a prevalence of 73.3% among those with type 2 diabetes [7, 8]. Furthermore, obese students with AN exhibit a markedly higher prevalence of hyperinsulinemia (46.2%) compared to those without either condition (1.4%) [9]. The presence of AN in children is closely associated with glucose intolerance, including impaired glucose tolerance and type 2 diabetes, with fasting plasma insulin concentrations exhibiting the strongest correlation [10]. Among adults aged 35–64 years, the overall prevalence of AN is 16.4%, escalating to 27.2 and 28.2% in males and females with metabolic syndrome [11].
This study aims to describe metabolic pathways underlying the development of AN and their clinical implications. While malignancies (such as gastric adenocarcinoma) can cause AN, this review focuses on the more common cause of AN, obesity, providing information on the relationships with insulin resistance, leptin and metabolic disorders and the potential for dermatologists to intervene to prevent cardiovascular comorbidity.
2 Pathophysiology
2.1 Role of insulin and insulin receptors
The development of AN is associated with obesity and diabetes mellitus, suggesting a potential role for hyperinsulinemia in the pathogenesis of the skin condition. AN mediators include insulin and its receptors, as well as insulin-like growth factor 1 (IGF1) and its receptors. IGF1, a hormone structurally similar to insulin, is primarily produced in the liver in response to growth hormone but can also be synthesized in other tissues, including fibroblasts in the skin [12, 13]. In normal physiological conditions, insulin binds with high affinity to the insulin receptor, exerting metabolic effects, while IGF1 binds with the highest affinity to the IGF1 receptor (IGF1R), promoting mitogenic effects [13]. However, in metabolically unhealthy individuals, elevated insulin levels can disrupt the balance and directly stimulate IGF1R on keratinocytes and dermal fibroblasts, strongly activating the Ras/MAP/ERK and PI3‐K/Akt kinase cascades and promoting cell proliferation, resulting in acanthosis, hyperkeratosis, and papillomatosis [12, 13]. Furthermore, high levels of insulin can not only directly stimulate IGF1 receptors but also reduce the synthesis of IGF binding proteins and displace IGF1 from its binding proteins, further enhancing IGF receptor activation [12, 14,15,16]. This direct stimulation of IGF1R and increased levels of IGF1 lead to fibroblast and keratinocyte proliferation, ultimately contributing to the development of AN (Fig. 1). AN developing at repeated insulin injection sites and in insulin-resistant syndromes further support this mechanism [17,18,19,20].
Acanthosis Nigricans and the insulin pathway. 1. At normal physiological levels insulin has high affinity for insulin receptors and induces metabolic effects. IGF1 has high affinity for IGF1R and has mitogenic effects. 2. At high levels insulin directly binds to IGF1R to cause mitogenic effects. 3. At high levels insulin decreases IGFBP synthesis and displaces IGF1 from IGFBP equally higher amounts of free IGF1 that can bind to IGFR1. 4. Once insulin or IGF1 binds to IGF1R it activates the Ras/MEK/ERK pathway and the P13k/Akt pathway. 5. This results in keratinocyte proliferation and Acanthosis Nigricans
2.2 Role of growth factor receptors
Another mechanism of AN involves the activation of fibroblast growth factor receptors (FGFR) and epidermal growth factor receptors (EGFR). FGFR, EGFR, and IGF1 are members of the same tyrosine kinase family and share similar downstream effects [12]. Certain malignancies, particularly gastric adenocarcinoma, may induce AN through a paraneoplastic phenomenon by stimulating these receptors. These malignancies result in increased levels of transforming growth factor alpha (TGF-α), which binds to EGFRs and promotes epidermal proliferation [15, 20,21,22]. Hereditary disorders have also been linked to AN as mutations in FGFR genes also contribute to this pathway. TGF-α, a mitogenic polypeptide belonging to the epidermal growth factor family, activates the classical MAPK/ERK pathway, the PI3‐K/Akt kinase pathway, and phospholipase (PL) C‐γ pathways when it binds to EGFR, leading to keratinocyte proliferation, differentiation, and migration [15, 21]. Binding of EGFR on keratinocytes can also trigger the production of self-ligands, including TNF-α, creating a feedforward autocrine cycle (Fig. 2). This mechanism may be interconnected with hyperinsulinemia, as high levels of insulin or IGF1 increase the binding of epidermal growth factor (EGF) to EGFR on keratinocytes, and evidence suggests cross talk between EGFR and IGF1R [23, 24]. These findings support the exploration of hyperinsulinemia as a potential contributor to the pathogenesis of AN.
Acanthosis Nigricans and the TNF-α pathway. Tumor cells secrete TNF-α resulting in increased serum levels. TNF-α binds to EGFR and activates the MAPK/ERK, P13/Akt, and PLC pathways, which leads to keratinocyte proliferation and AN. High insulin levels increase the binding of EGF on EGFR resulting in more activation and more keratinocyte proliferation
2.3 Role of leptin
Leptin is also involved in skin proliferation and obesity-related skin disorders. Leptin, encoded by the Obese (Ob) gene, is a protein hormone primarily secreted by adipose cells, with its production directly proportional to the number of adipocytes in the body [25,26,27]. While its most well-known function is appetite suppression and energy homeostasis, leptin has other effects. Under normal physiological conditions, leptin circulates through the bloodstream and crosses the blood–brain barrier to bind to leptin receptors in the hypothalamus [25,26,27,28]. Binding of leptin to the long form of its receptor (LepRb or ObRb) in the hypothalamus activates the tyrosine protein kinase JAK2 [28, 29]. This activation leads to the phosphorylation of tyrosine and activation of the MAPK, STAT5, and STAT3 pathways resulting in the regulation of food intake and energy homeostasis [25, 28].
Leptin also plays a role in insulin resistance as it interacts with insulin receptor substrate 1 (IRS1) and IRS2, promoting insulin sensitivity and reducing insulin levels [30]. However, during obesity, the elevated leptin levels can act as a pro-inflammatory cytokine, potentially leading to insulin resistance [31, 32]. Insulin resistance leads to increased insulin levels, which, in turn, further enhances leptin synthesis and release [29]. This creates a feedforward mechanism involving obesity, leptin, and insulin, resulting in a state of hyperinsulinemia and hyperleptinemia (Fig. 3).
Acanthosis Nigricans and the development of hyperinsulinemia and hyperleptinemia. The development of obesity can result in insulin resistance, which results in increased serum levels of insulin. Insulin increases leptin production and leptin receptors throughout the body. Elevated levels of leptin can further develop insulin resistance resulting in a state of hyperinsulinemia and hyperleptinemia
While leptin and receptors are produced in adipocytes, they are also expressed in various other tissues, including keratinocytes and fibroblasts [33,34,35,36,37]. The leptin receptors in these skin cells are similar to those in adipocytes and the hypothalamus. Insulin increases the expression of leptin receptors in fibroblasts, suggesting that leptin may have autocrine and paracrine functions [33, 34]. Leptin can stimulate the proliferation of keratinocytes and fibroblasts, promote epithelialization, and enhance collagen synthesis by binding to ObRb receptors in the skin and modulating the JAK/STAT (Fig. 4), and ERK pathways [33, 35, 36]. Additionally, leptin receptors in AN-affected tissue are upregulated [37]. The increased proliferation induced by leptin, along with the proliferation stimulated by IGFR, likely contributes to the development of AN. These findings are supported by the positive correlation between increased leptin levels and AN compared to obesity alone [38,39,40,41].
Acanthosis Nigricans and the leptin signaling pathway. 1. Adipocytes are the main source of Leptin synthesis, but many other tissues synthesize leptin including fibroblasts and keratinocytes. 2. Insulin increases leptin synthesis and leptin receptors (ObRb) in the tissues. Leptin in turn decreases insulin synthesis and secretion from the pancreatic beta cells. 3. Leptin binds to immune cells causing them to release proinflammatory markers. In turn these stimulate adipocytes to synthetize more leptin. 4. Leptin binds to Leptin receptors (ObRb) on keratinocytes. 5. The binding of leptin activates JAK2 Tyrosine Kinases which then phosphorylate STAT3. 6. The phosphorylated STAT3s dimerize and transport to the nucleus to regulate their target genes
3 Treatment (Table 1)
The primary approach to treating AN is addressing the root cause. As most cases of AN are associated with obesity, hyperinsulinemia, and hyperleptinemia, weight loss is effective in reducing the severity of the disease [20, 42, 43]. Weight loss improves insulin resistance, leading to decreased insulin levels and a reduction in inflammatory markers such as TNF-α [44]. Additionally, weight loss results in decreased adipocytes, which subsequently lowers leptin levels as there is a direct correlation between leptin levels and obesity [25,26,27]. Another potential approach worth considering is focused on reducing leptin levels in patients. Lowering plasma leptin levels restores leptin sensitivity in the hypothalamus, leading to improved insulin sensitivity and weight loss [45]. Although there are currently no approved therapies targeting leptin levels for AN, ongoing research may shed light on potential future treatments.
Topical treatments have been a first-line therapy for AN. Retinoids, vitamin D analogs, keratolytic creams, and chemical peels are commonly used, with retinoids being the most utilized [20, 46]. Various retinoids are effective in treating AN lesions, but continuous use is often required to prevent relapse [47]. In case reports, topical vitamin D analogs were effective in treating AN, as vitamin D reduces keratinocyte proliferation and may, therefore, be beneficial in hyperproliferative skin diseases [48,49,50,51].
Chemical peels work by destroying the epidermis, which then requires repair and rejuvenation of the skin [20]. Chemical peels with trichloroacetic acid decrease papillomatosis and hyperpigmentation within one month of use and show improvement in the appearance of AN [52]. Other peels, including glycolic acid and salicylic acid, also show improvement, but may not be effective as other therapies [53].
Laser treatments have emerged as a promising option for AN management and are more effective than topical therapies [54,55,56,57]. Lasers can be ablative or non-ablative, and they can also be fractionated or non-fractionated. Ablative lasers are the most aggressive as they remove the epidermal skin layer, resulting in more dramatic treatment effects [58]. Non-fractionated lasers treat the entire projected surface area of the skin, whereas fractionated lasers treat smaller sections of skin within a target area [58]. The ablative fractional carbon dioxide laser is very effective in treating AN and is more effective than chemical peels and creams [54, 56]. Non-ablative lasers, such as the long-pulsed alexandrite laser and fractional 1550-nm erbium fiber laser, are not as aggressive as the carbon dioxide laser, but are also effective in treating AN lesions [20, 53]. While laser therapy is effective, it can be costly, and multiple treatments may be necessary.
Systemic treatments for AN are generally reserved for lesions that are resistant to topical treatments or those that are too extensive for localized therapy. Oral therapies, such as isotretinoin, metformin, and melatonin, have been explored with varying results [19]. Isotretinoin and related oral retinoids have similar results to topical retinoids, with lesions improving during treatment but relapsing after discontinuation [47, 59]. However, due to the numerous side effects associated with oral retinoids, they are generally not considered useful for treating AN.
Metformin and other insulin sensitizers have some effectiveness in the treatment of AN associated with insulin resistance and obesity. These medications work by reducing glucose levels and reduce the number of AN skin lesions [47, 60]. In a recent small double-blind randomized controlled trial comparing metformin to an OTC combination medicine of alpha-lipoic acid, biotin, calcium pantothenate, and zinc sulfate for the treatment of AN, both medications improved the AN lesions [61]. Considering metformin's safety profile and widespread use for diabetes management, it is a reasonable option for the additional benefit of treating AN, especially in obese patients.
Other diabetic drugs may also hold promise for AN. Thiazolidinediones effectively lower insulin levels and are more effective in treating diabetes compared to metformin [62]. While thiazolidinediones have not been extensively studied specifically for AN treatment, there is evidence suggesting their potential benefit. A combination treatment of DPP-4 inhibitors and thiazolidinediones improve AN lesions [63].
Glucagon-like peptide-1 (GLP-1) receptor agonists may also be useful for AN treatment. These agents improve weight loss and insulin resistance, both of which contribute to the pathogenesis of AN [64]. The benefit of enhancing glycemic control and promoting weight loss makes GLP-1 receptor agonists a promising option for managing AN in patients with diabetes. Further research is needed, however, as we identified no studies on the subject.
4 Discussion
4.1 Differential diagnoses
There are typical findings in AN that can be quickly recognized. The velvety hyperpigmented and hyperkeratotic lesions appear mostly in the axillae, neck, and body folds [20]. There can also be atypical locations including the scalp, face, hands, over joints and anywhere else on the body [20]. At times the lesions can mimic the appearance of psoriasis and with psoriasis being tied to metabolic syndrome, obese patients may present with both diseases. Psoriasis can be differentiated from AN by its raised, well-demarcated lesions that are typically erythematous and more inflamed. Another mimic may be terra firma-forme dermatosis, which appears as dirt-like lesions, typically on the face, neck, and ankles. The lesions can easily be distinguished from AN by treating by rubbing with alcohol wipes [65]. Other lesions that can mimic AN include, melasma, atopic dermatitis, lichen simplex chronicus, post-inflammatory hyperpigmentation, and seborrheic keratosis, and several others [20].
Rarely AN can be a cutaneous manifestation of various malignancies, particularly gastric adenocarcinoma [1, 20]. Malignant AN can present similarly to benign AN, but malignant AN can have a more abrupt onset, involve more of the oral cavity, and have intense pruritus [1]. This should be a high suspicion, especially when the patient presents with constitutional symptoms and AN; however, malignant AN can present before any clinical signs of malignancy appear.
5 Metabolic syndrome
AN is positively correlated with meeting the criteria for metabolic syndrome of hyperglycemia, high BMI, increased waist circumference, high triglycerides, and hypertension [11]. Meeting 3 of the 5 criteria for metabolic syndrome doubles the risk of cardiovascular disease and increases all-cause mortality [66]. The quick recognition of AN’s significance in cardiovascular risk assessment could aid in the early detection and prevention of cardiovascular diseases, leading to improved patient outcomes.
AN has potential as an early screening tool for metabolic diseases such as insulin resistance and subclinical atherosclerosis [42]. Given the association of AN with high levels of insulin, BMI, and leptin, there is an increased risk for cardiovascular diseases. Insulin has negative cardiovascular implications as insulin resistance can increase the risk of myocardial infarction by 2 to 4 times [67]. Similar to how insulin and IGF lead to changes in the skin, high levels of insulin can bind to receptors on the heart and lead to cardiovascular remodeling and heart failure [68]. Similarly, insulin resistance can have negative effects on the vasculature. Under normal conditions, Insulin binds to vasculature endothelium receptors, resulting in nitric oxide release for vasodilation [69]. Insulin resistance disrupts this process, causing adverse inflammatory effects such as mitochondrial dysfunction, oxidative stress, and protein kinase C activation which leads to endothelial dysfunction, impaired vasodilation, arterial narrowing, and atherosclerosis [69].
5.1 Screening for metabolic and cardiovascular risk factors
Considering that AN screening is easily performed and noninvasive, it could serve as an affordable and useful screening tool. AN has already been used as a screening tool in children and adolescents to identify those at risk of developing type 2 diabetes [9, 70, 71]. This screening could be further expanded to include cardiovascular disease. Presentation of AN could warrant referral to primary care for measurements of blood pressure, BMI, waist circumference, glucose levels, and a lipid panel which are all determinants of metabolic syndrome. Measurements of insulin resistance could also be considered, including homeostasis model assessment of insulin resistance (HOMA-IR), fasting insulin level, glucose/insulin ratio, etc. By combining the simplicity of AN screening with a comprehensive assessment of metabolic and cardiovascular risk factors, patients will have improved health outcomes as many patients presenting to dermatology have many unmet preventative healthcare needs [72].
6 Conclusion
AN is not simply a cosmetic issue. When patients present with AN it may be an indication that they may benefit from referring to a primary care to discuss screening for metabolic disease, exercise, diet, and the cardiovascular risks associated with AN. Treatment options attacking the root cause of AN, including improving metabolic health and weight loss are effective in treating the disease [21, 46, 47]. Cosmetic treatments have been used with good but temporary results. Newer treatments like SGLT2 inhibitors may show promise for treating AN as it helps with insulin resistance, obesity, and improves cardiovascular outcomes. Further research is needed, however, on the specific effects of SGLT2 inhibitors on AN as we identified no studies on the subject.
Data availability
No datasets were generated or analysed during the current study.
References:
Karadağ AS, You Y, Danarti R, Al-Khuzaei S, Chen W. Acanthosis nigricans and the metabolic syndrome. Clin Dermatol. 2018;36(1):48–53. https://doi.org/10.1016/j.clindermatol.2017.09.008.
Radu AM, Carsote M, Dumitrascu MC, Sandru F. Acanthosis nigricans: pointer of endocrine entities. Diagnostics (Basel). 2022;12(10):2519. https://doi.org/10.3390/diagnostics12102519.
Stuart CA, Pate CJ, Peters EJ. Prevalence of acanthosis nigricans in an unselected population. Am J Med. 1989;87(3):269–72. https://doi.org/10.1016/s0002-9343(89)80149-4.
Sudevan R, Vijay Kumar S, Sunny C, Sunand N, Vasudevan A. Prevalence of Acanthosis nigricans and its association with physical activity in adolescents - School-based analytical cross-sectional study from Kochi, Kerala. J Family Med Prim Care. 2021;10(11):4218–22. https://doi.org/10.4103/jfmpc.jfmpc_953_21.
Hud JA, Cohen JB, Wagner JM, Cruz PD. Prevalence and significance of Acanthosis nigricans in an adult obese population. Arch Dermatol. 1992;128(7):941–4. https://doi.org/10.1001/archderm.1992.01680170073009.
Stuart CA, Smith MM, Gilkison CR, Shaheb S, Stahn RM. Acanthosis nigricans among Native Americans: an indicator of high diabetes risk. Am J Public Health. 1994;84(11):1839–42. https://doi.org/10.2105/ajph.84.11.1839.PMID:7977931;PMCID:PMC1615192.
Copeland K, Pankratz K, Cathey V, Immohotichey P, Maddox J, Felton B, McIntosh R, Parker D, Burgin C, Blackett P. Acanthosis nigricans, insulin resistance (HOMA) and dyslipidemia among Native American children. J Okla State Med Assoc. 2006;99(1):19–24.
Stoddart ML, Blevins KS, Lee ET, Wang W, Blackett PR, Cherokee Diabetes Study. Association of Acanthosis nigricans with hyperinsulinemia compared with other selected risk factors for type 2 diabetes in Cherokee Indians: the Cherokee Diabetes Study. Diabetes Care. 2002;25(6):1009–14. https://doi.org/10.2337/diacare.25.6.1009.
Mukhtar Q, Cleverley G, Voorhees RE, McGrath JW. Prevalence of Acanthosis nigricans and its association with hyperinsulinemia in New Mexico adolescents. J Adolesc Health. 2001;28(5):372–6. https://doi.org/10.1016/s1054-139x(00)00217-2.
Yamazaki H, Ito S, Yoshida H. Acanthosis nigricans is a reliable cutaneous marker of insulin resistance in obese Japanese children. Pediatr Int. 2003;45(6):701–5. https://doi.org/10.1111/j.1442-200x.2003.01812.x.
Dassanayake AS, Kasturiratne A, Niriella MA, et al. Prevalence of Acanthosis nigricans in an urban population in Sri Lanka and its utility to detect metabolic syndrome. BMC Res Notes. 2011;4:25. https://doi.org/10.1186/1756-0500-4-25.
Torley D, et al. Genes, growth factors and Acanthosis nigricans. Br J Dermatol. 2002;147(6):1096–101. https://doi.org/10.1046/j.1365-2133.2002.05150.x.
Farag AGA, Abdu Allah AMK, El-Rebey HS, Mohamed Ibraheem KI, Mohamed ASED, Labeeb AZ, Elgazzar AE, Haggag MM. Role of insulin-like growth factor-1 in skin tags: a clinical, genetic and immunohistochemical study in a sample of Egyptian patients. Clin Cosmet Investig Dermatol. 2019;26(12):255–66. https://doi.org/10.2147/CCID.S192964.PMID:31118729;PMCID:PMC6503204.
Cai W, Sakaguchi M, Kleinridders A, Gonzalez-Del Pino G, Dreyfuss JM, O’Neill BT, Ramirez AK, Pan H, Winnay JN, Boucher J, Eck MJ, Kahn CR. Domain-dependent effects of insulin and IGF-1 receptors on signalling and gene expression. Nat Commun. 2017;27(8):14892. https://doi.org/10.1038/ncomms14892.
Ando Y, Jensen PJ. Epidermal growth factor and insulin-like growth factor I enhance keratinocyte migration. J Invest Dermatol. 1993;100(5):633–9. https://doi.org/10.1111/1523-1747.ep12472297.
Nam SY, Lee EJ, Kim KR, Cha BS, Song YD, Lim SK, Lee HC, Huh KB. Effect of obesity on total and free insulin-like growth factor (IGF)-1, and their relationship to IGF-binding protein (BP)-1, IGFBP-2, IGFBP-3, insulin, and growth hormone. Int J Obes Relat Metab Disord. 1997;21(5):355–9. https://doi.org/10.1038/sj.ijo.0800412.
Vuillamy C, Arnault JP, Attencourt C, Dadban A, Joachim C, Chaby G, Lok C. Simultaneous occurrence of insulin-derived amyloidosis and Acanthosis nigricans at the abdominal site of insulin injection. JAAD Case Rep. 2021;11(19):94–6. https://doi.org/10.1016/j.jdcr.2021.11.003.
Godse R, Rauck C, Woods R, Steele KT, Elenitsas R. Two cases of insulin-derived amyloidosis with Acanthosis nigricans-like changes. Am J Dermatopathol. 2022;44(12):979–80. https://doi.org/10.1097/DAD.0000000000002314.
Huang Y, Hessami-Booshehri M. Acanthosis nigricans at sites of insulin injection in a man with diabetes. CMAJ. 2018;190(47):E1390. https://doi.org/10.1503/cmaj.180705.PMID:30478218;PMCID:PMC6246042.
Leung AKC, Lam JM, Barankin B, Leong KF, Hon KL. Acanthosis nigricans: an updated review. Curr Pediatr Rev. 2022;19(1):68–82. https://doi.org/10.2174/1573396318666220429085231.
Koyama S, Ikeda K, Sato M, Shibahara K, Yuhara K, Fukutomi H, Fukunaga K, Kanazawa N, Yuzawa K, Fukao K, Iijima T, Kikuchi M, Tomiya T, Fujiwara K. Transforming growth factor-alpha (TGF alpha)-producing gastric carcinoma with Acanthosis nigricans: an endocrine effect of TGF alpha in the pathogenesis of cutaneous paraneoplastic syndrome and epithelial hyperplasia of the esophagus. J Gastroenterol. 1997;32(1):71–7. https://doi.org/10.1007/BF01213299.
Haase I, Hunzelmann N. Activation of epidermal growth factor receptor/ERK signaling correlates with suppressed differentiation in malignant Acanthosis nigricans. J Invest Dermatol. 2002;118(5):891–3. https://doi.org/10.1046/j.1523-1747.2002.17631.x.
Krane JF, Murphy DP, Carter DM, Krueger JG. Synergistic effects of epidermal growth factor (EGF) and insulin-like growth factor I/somatomedin C (IGF-I) on keratinocyte proliferation may be mediated by IGF-I transmodulation of the EGF receptor. J Invest Dermatol. 1991;96(4):419–24. https://doi.org/10.1111/1523-1747.ep12469799.
Roudabush FL, Pierce KL, Maudsley S, Khan KD, Luttrell LM. Transactivation of the EGF receptor mediates IGF-1-stimulated SHC phosphorylation and ERK1/2 activation in COS-7 cells. J Biol Chem. 2000;275(29):22583–9. https://doi.org/10.1074/jbc.M002915200.
Obradovic M, Sudar-Milovanovic E, Soskic S, Essack M, Arya S, Stewart AJ, Gojobori T, Isenovic ER. Leptin and obesity: role and clinical implication. Front Endocrinol (Lausanne). 2021;18(12): 585887. https://doi.org/10.3389/fendo.2021.585887.
Fried SK, Ricci MR, Russell CD, Laferrère B. Regulation of leptin production in humans. J Nutr. 2000;130(12):3127S-3131S. https://doi.org/10.1093/jn/130.12.3127S.
Pan W, Myers M. Leptin and the maintenance of elevated body weight. Nat Rev Neurosci. 2018;19:95–105. https://doi.org/10.1038/nrn.2017.168.
Zhao S, Kusminski CM, Elmquist JK, Scherer PE. Leptin: less is more. Diabetes. 2020;69(5):823–9. https://doi.org/10.2337/dbi19-0018.PMID:32312898;PMCID:PMC7171955.
Harris RB. Direct and indirect effects of leptin on adipocyte metabolism. Biochim Biophys Acta. 2014;1842(3):414–23. https://doi.org/10.1016/j.bbadis.2013.05.009.
Amitani M, Asakawa A, Amitani H, Inui A. The role of leptin in the control of insulin-glucose axis. Front Neurosci. 2013;8(7):51. https://doi.org/10.3389/fnins.2013.00051.PMID:23579596;PMCID:PMC3619125.
Kumar R, Mal K, Razaq MK, Magsi M, Memon MK, Memon S, Afroz MN, Siddiqui HF, Rizwan A. Association of leptin with obesity and insulin resistance. Cureus. 2020;12(12): e12178. https://doi.org/10.7759/cureus.12178.PMID:33489589;PMCID:PMC7815269.
López-Jaramillo P, Gómez-Arbeláez D, López-López J, López-López C, Martínez-Ortega J, Gómez-Rodríguez A, Triana-Cubillos S. The role of leptin/adiponectin ratio in metabolic syndrome and diabetes. Horm Mol Biol Clin Invest. 2014;18(1):37–45. https://doi.org/10.1515/hmbci-2013-0053.
Dopytalska K, Baranowska-Bik A, Roszkiewicz M, Bik W, Walecka I. The role of leptin in selected skin diseases. Lipids Health Dis. 2020;19(1):215. https://doi.org/10.1186/s12944-020-01391-8.
Glasow A, Kiess W, Anderegg U, Berthold A, Bottner A, Kratzsch J. Expression of leptin (Ob) and leptin receptor (Ob-R) in human fibroblasts: regulation of leptin secretion by insulin. J Clin Endocrinol Metab. 2001;86(9):4472–9. https://doi.org/10.1210/jcem.86.9.7792.
Frank S, Stallmeyer B, Kämpfer H, Kolb N, Pfeilschifter J. Leptin enhances wound re-epithelialization and constitutes a direct function of leptin in skin repair. J Clin Invest. 2000;106(4):501–9. https://doi.org/10.1172/JCI9148.
Yuan C, Liao J, Zheng L, Ding L, Teng X, Lin X, Wang L. Current knowledge of leptin in wound healing: a collaborative review. Front Pharmacol. 2022;12(13): 968142. https://doi.org/10.3389/fphar.2022.968142.
Mohammed EE, Mohammed RR, Elhakeem AA, Riad AA. Leptin receptor expression in obesity-associated Acanthosis nigricans. Minia J Med Res. 2020;31(2):308–12. https://doi.org/10.21608/mjmr.2022.221080.
Atwa M, Emara A, Balata M, Youssef N, Bayoumy N, Sherif A, Fiala L. Serum leptin, adiponectin, and resistin among adult patients with Acanthosis nigricans: correlations with insulin resistance and risk factors for cardiovascular disease. Int J Dermatol. 2014;53(10):e410–20. https://doi.org/10.1111/ijd.12340.
Agrawal K, Mathur R, Purwar N, Mathur SK, Mathur DK. Hyperandrogenism, insulin resistance, and Acanthosis nigricans (HAIR-AN) syndrome reflects adipose tissue dysfunction (“Adiposopathy” or “Sick Fat”) in Asian Indian Girls. Dermatology. 2021;237(5):797–805. https://doi.org/10.1159/000512918.
Wang Y, Liu X, Wang Z, Li Y, Chen T, Li B, Zhai N, Li J. Leptin, adiponectin and sex hormone in benign Acanthosis nigricans of males. Zhonghua Yi Xue Za Zhi. 2014;94(44):3475–7.
Huang Y, Chen J, Wang X, Li Y, Yang S, Qu S. The clinical characteristics of obese patients with Acanthosis nigricans and its independent risk factors. Exp Clin Endocrinol Diabetes. 2017;125(3):191–5. https://doi.org/10.1055/s-0042-123035.
Kutlubay Z, Engin B, Bairamov O, Tüzün Y. Acanthosis nigricans: a fold (intertriginous) dermatosis. Clin Dermatol. 2015;33(4):466–70. https://doi.org/10.1016/j.clindermatol.2015.04.010.
Danda VSR, Srinivas Rao P, Konda C, Lodha P. Acanthosis nigricans in Insulinoma: reversible experiments of the nature. Med J Armed Forces India. 2022;78(Suppl 1):S315–8. https://doi.org/10.1016/j.mjafi.2019.06.002.
Unluhizarci K, Karaca Z, Kelestimur F. Role of insulin and insulin resistance in androgen excess disorders. World J Diabetes. 2021;12(5):616–29. https://doi.org/10.4239/wjd.v12.i5.616.
Zhao S, Zhu Y, Schultz RD, Li N, He Z, Zhang Z, Caron A, Zhu Q, Sun K, Xiong W, Deng H, Sun J, Deng Y, Kim M, Lee CE, Gordillo R, Liu T, Odle AK, Childs GV, Zhang N, Kusminski CM, Elmquist JK, Williams KW, An Z, Scherer PE. Partial leptin reduction as an insulin sensitization and weight loss strategy. Cell Metab. 2019;30(4):706-719.e6. https://doi.org/10.1016/j.cmet.2019.08.005.
Treesirichod A, Chaithirayanon S, Wongjitrat N. Comparison of the efficacy and safety of 0.1% adapalene gel and 0.025% tretinoin cream in the treatment of childhood Acanthosis nigricans. Pediatr Dermatol. 2019;36(3):330–4. https://doi.org/10.1111/pde.13799.
Phiske MM. An approach to Acanthosis nigricans. Indian Dermatol Online J. 2014;5(3):239–49. https://doi.org/10.4103/2229-5178.137765.
Liang W, Lin Z, Zhang L, Qin X, Zhang Y, Sun L. Calcipotriol inhibits proliferation of human keratinocytes by downregulating STAT1 and STAT3 signaling. J Investig Med. 2017;65(2):376–81. https://doi.org/10.1136/jim-2016-000176.
Takahashi H, Ibe M, Kinouchi M, Ishida-Yamamoto A, Hashimoto Y, Iizuka H. Similarly potent action of 1,25-dihydroxyvitamin D3 and its analogues, tacalcitol, calcipotriol, and maxacalcitol on normal human keratinocyte proliferation and differentiation. J Dermatol Sci. 2003;31(1):21–8. https://doi.org/10.1016/s0923-1811(02)00136-6.
Gregoriou S, Anyfandakis V, Kontoleon P, Christofidou E, Rigopoulos D, Kontochristopoulos G. Acanthosis nigricans associated with primary hypogonadism: successful treatment with topical calcipotriol. J Dermatolog Treat. 2008;19(6):373–5. https://doi.org/10.1080/09546630802050506.
Lee HW, Chang SE, Lee MW, Choi JH, Moon KC, Koh JK. Hyperkeratosis of the nipple associated with Acanthosis nigricans: treatment with topical calcipotriol. J Am Acad Dermatol. 2005;52(3 Pt 1):529–30. https://doi.org/10.1016/j.jaad.2004.09.028.
Baldissera RL, Yang EJ, Schmitt JV, et al. Trichloroacetic acid peels for the treatment of acanthosis Nigricans. J Am Acad Dermatol. 2022;86(1):203–4. https://doi.org/10.1016/j.jaad.2021.01.065.52.
Zeeshan M, Arfeen N, Sonthalia S, Singh A, Roy PK. Treatment of acanthosis nigricans with sequential salicylic acid-mandelic acid combination peel and maintenance with glycolic acid-urea combination cream: a retrospective pilot study. J Cosmet Dermatol. 2022;21(9):3905–9. https://doi.org/10.1111/jocd.14731.
Abu Oun AA, Ahmed NA, Hafiz HSA. Comparative study between fractional carbon dioxide laser versus retinoic acid chemical peel in the treatment of Acanthosis nigricans. J Cosmet Dermatol. 2022;21(3):1023–30. https://doi.org/10.1111/jocd.14224.
Ehsani A, Noormohammadpour P, Goodarzi A, Mirshams Shahshahani M, Hejazi SP, Hosseini E, Azizpour A. Comparison of long-pulsed alexandrite laser and topical tretinoin-ammonium lactate in axillary Acanthosis nigricans: a case series of patients in a before-after trial. Caspian J Intern Med. 2016;7(4):290–3.
Campos MA, Varela P, Baptista A, Ferreira EO. Unilateral nevoid Acanthosis nigricans treated with CO2 laser. BMJ Case Rep. 2016. https://doi.org/10.1136/bcr-2016-216073.
Eldeeb F, Wahid RM, Alakad R. Fractional carbon dioxide laser versus trichloroacetic acid peel in the treatment of pseudo-acanthosis Nigricans. J Cosmet Dermatol. 2022;21(1):247–53. https://doi.org/10.1111/jocd.14088.
Preissig J, Hamilton K, Markus R. Current laser resurfacing technologies: a review that delves beneath the surface. Semin Plast Surg. 2012;26(3):109–16. https://doi.org/10.1055/s-0032-1329413.
Higgins SP, Freemark M, Prose NS. Acanthosis nigricans: a practical approach to evaluation and management. Dermatol Online J. 2008;14(9):2.
Giri D, Alsaffar H, Ramakrishnan R. Acanthosis nigricans and Its response to metformin. Pediatr Dermatol. 2017;34(5):e281–2. https://doi.org/10.1111/pde.13206.
Sett A, Pradhan S, Sancheti K, Basu D, Datta A, Biswas L, Das S, Pal SK, Gupta N, Sil A, Das NK. Effectiveness and safety of metformin versus canthex™ in patients with Acanthosis nigricans: a randomized, double-blind controlled trial. Indian J Dermatol. 2019;64(2):115–21. https://doi.org/10.4103/ijd.IJD_417_17.
Bellot-Rojas P, Posadas-Sanchez R, Caracas-Portilla N, et al. Comparison of metformin versus rosiglitazone in patients with Acanthosis nigricans: a pilot study. J Drugs Dermatol. 2006;5(9):884–9.
Adderley-Rolle EM, Peter S. Regression of Acanthosis nigricans with the addition of sitagliptin and pioglitazone. West Indian Med J. 2015;64(2):160–1. https://doi.org/10.7727/wimj.2013.334.
Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes—state-of-the-art. Mol Metab. 2021;46: 101102. https://doi.org/10.1016/j.molmet.2020.101102.
Sechi A, Patrizi A, Savoia F, Leuzzi M, Guglielmo A, Neri I. Terra firma-forme dermatosis: a systematic review. Int J Dermatol. 2021;60(8):933–43. https://doi.org/10.1111/ijd.15301.
Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56(14):1113–32. https://doi.org/10.1016/j.jacc.2010.05.034.
Laakso M, Lehto S. Epidemiology of risk factors for cardiovascular disease in diabetes and impaired glucose tolerance. Atherosclerosis. 1998;137(Suppl):S65-73. https://doi.org/10.1016/s0021-9150(97)00314-6.
Riehle C, Abel ED. Insulin signaling and heart failure. Circ Res. 2016;118(7):1151–69. https://doi.org/10.1161/CIRCRESAHA.116.306206.
Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113(15):1888–904. https://doi.org/10.1161/CIRCULATIONAHA.105.563213.
Rodríguez-Gutiérrez R, de la O-Cavazos ME, Salcido-Montenegro A, Sanchez-Garcia A, Gomez-Flores M, Gonzalez-Nava V, Castillo-Gonzalez D, Santos-Santillana KM, González-González JG. Acanthosis Nigricans in the knuckles of infants: a novel clinical marker of high metabolic risk. Diabetes Ther. 2019;10(6):2169–2181. https://doi.org/10.1007/s13300-019-00703-1.
Bhagyanathan M, Dhayanithy D, Parambath VA, Bijayraj R. Acanthosis Nigricans: a screening test for insulin resistance—an important risk factor for diabetes mellitus type-2. J Family Med Prim Care. 2017;6(1):43–6. https://doi.org/10.4103/2249-4863.214961.
Feldman SR, Ravis S, Moran WP, Fleischer AB Jr. Patients seen in a dermatology clinic have unmet preventive health care needs. J Am Acad Dermatol. 2001;44(4):706–9. https://doi.org/10.1067/mjd.2001.112914.
Funding
None.
Ethics declarations
Competing interests
Feldman has received research, speaking and/or consulting support from Eli Lilly and Company, GlaxoSmithKline/Stiefel, AbbVie, Janssen, Alovtech, vTv Therapeutics, Bristol-Myers Squibb, Samsung, Pfizer, Boehringer Ingelheim, Amgen, Dermavant, Arcutis, Novartis, Novan, UCB, Helsinn, Sun Pharma, Almirall, Galderma, Leo Pharma, Mylan, Celgene, Ortho Dermatology, Menlo, Merck & Co, Qurient, Forte, Arena, Biocon, Accordant, Argenx, Sanofi, Regeneron, the National Biological Corporation, Caremark, Teladoc, BMS, Ono, Micreos, Eurofins, Informa, UpToDate and the National Psoriasis Foundation. He is founder and part owner of Causa Research and holds stock in Sensal Health. The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eggiman, E., Feldman, S.R. The underlying pathogenesis of obesity-associated acanthosis nigricans: a literature review. Discov Med 1, 19 (2024). https://doi.org/10.1007/s44337-024-00017-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44337-024-00017-7