Abstract
Splenic artery aneurysm (SAA) is the most common aneurysm of the visceral arteries. SAA can be silent and asymptomatic; rupture is a rare but serious complication that can manifest as acute diffuse abdominal pain and hypovolemic shock with a high risk of mortality. Surgery is the traditional treatment, but has the disadvantage of severe surgical injuries, a high risk of complications and a high mortality rate. We present the case of a 58-year-old woman with a saccular aneurysm of the middle segment of the splenic artery; she was treated by coiling embolization using the sandwich technique. Contrast-enhanced computed tomography (CECT) controls at one month, four months and one year showed progressive reduction and complete devascularization of the aneurysmal sac. Embolization of the splenic artery aneurysm is an effective and safe treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Splenic artery aneurysm (SAA) is defined as a focal dilatation in the diameter of the splenic artery, 50% greater than the normal vessel diameter.
The prevalence of SAA in the general population is reported to be less than 1%, as most SAAs remain asymptomatic and therefore undetected. A recent retrospective study found that 78% of SAAs occur in women. Mortality due to rupture of the splenic aneurysm in non-pregnant patients is between 25 and 40%. However, maternal mortality due to SAA rupture increases up to 75% and fetal mortality up to 95% [1, 2]
The clinical presentation of SAA is variable, with most splenic artery aneurysms being asymptomatic and discovered incidentally.
However, aneurysms that are symptomatic or greater than 2 cm in diameter are at increased risk of rupture, being associated with higher mortality.
Typical clinical symptoms of a ruptured splenic artery aneurysm are abdominal pain in the left upper quadrant and hemodynamic instability.
Treatment should be considered if a larger aneurysm (> 2 cm) is present. A rapidly growing and symptomatic aneurysm may warrant intervention regardless of its size.
In the past, splenic artery aneurysm has been treated surgically (ligation of the splenic artery, ligation of the aneurysm or aneurysmectomy with or without splenectomy, depending on the location of the aneurysm) [3].
The surgical procedure is associated with various complications; endovascular treatments have the lowest mortality rate compared to open and laparoscopic procedures.
We report a case of SAA treated with coiling embolization using the sandwich technique.
Case presentation
A 58-year-old female patient with occasional abdominal pain underwent abdominal ultrasonography (US), which revealed a suspected iso-hyperechogenic pancreatic formation. The laboratory tests were normal.
So she presented to our hospital for investigation.
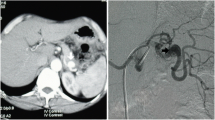
Abdominal Contrast-enhanced Computed Tomography (CECT) showed a saccular aneurysm (45 × 44x42 mm) with eccentric parietal thrombosis in the middle segment of the splenic artery. (Fig. 1a, b).
The case was referred to our Department of Interventional Radiology for treatment.
Due to the size and high risk of bleeding, it was decided to perform endovascular embolization treatment.
First, in a sterile environment and after local anesthesia, the right femoral access was achieved using a 18 Gauge (G) needle and a 5 French (Fr) sheath was inserted using the Seldinger technique. Second, the celiac trunk was selectively catheterized with a 5 Fr C1 catheter; arteriography confirmed the presence of the larger saccular splenic aneurysm. (Fig. 2a).
The efferent branch, the aneurysmal sac and the afferent arterial branch were catheterized superselectively with a 2.7 Fr microcatheter. Embolization of the aneurysm was performed using a sandwich technique with the release of POD (6 × 500 mm) in the efferent branch, six coils in the aneurysmal sac and one coil (8 × 300 mm) in the afferent branch. (Fig. 2b).
The control Cone-beam Computed Tomography (CBCT) control showed a good procedural result with devascularization of the aneurysm, hypertrophy of the left gastric artery and opacification of several gastro-splenic collaterals, without peri- and post-procedural complications.
Hemostasis of the access site is performed with a special device and manual compression for 20 min.
Abdominal ultrasonography (US) one day later showed the complete devascularization of the aneurysm and preserved vascularization of the spleen with the presence of only a very small ischemic area without serious complications.
The control Contrast-Enhanced Computed Tomography (CECT) after one, three and twelve months showed complete devascularization and progressive reduction of the splenic aneurysmal sac with preserved vascularization of the spleen, without any serious complications. Figure 3a, b
Discussion
Splenic artery aneurysm (SAA) is a rare but seriously life-threatening vascular disease [4].
The significance of splenic artery aneurysm lies in the potential risk of rupture and hemorrhage, which occurs in 10% of cases with a mortality rate of 10–25% in non-pregnant patients and up to 70% during pregnancy [5,6,7,8].
Computed tomography angiography (CTA) is the first diagnostic tool of choice for SAAs.
In patients with suspected SAAs and pre-existing renal insufficiency, which limits the use of iodinated contrast media, magnetic resonance angiography (MRA) is used to confirm the diagnosis.
Arteriography is recommended if non-invasive examinations have not sufficiently demonstrated the status of the relevant collateral blood flow and if endovascular intervention is planned.
The indications for intervention in SAAs are related to factors that may increase the risk of spontaneous rupture, including [9]: Asymptomatic SAAs with a diameter ≥ 2 cm, SAAs with a diameter < 2 cm but gradually increasing during follow-up, SAAs of any size in women of childbearing age or during pregnancy, and in patients with portal hypertension.
Traditional treatment of SAA involves open or laparoscopic surgery to resect the spleen or ligate the blood vessels. In general, aneurysmectomy and reconstruction with preservation of the spleen is the option chosen for proximal SAA, while distal SAA requires aneurysmectomy with splenectomy.
Simple laparoscopic ligature of the artery proximal to the SAA combined with resection of the SAA with or without splenectomy has been increasingly employed.
These methods are traumatic and have more complications, slower postoperative recovery and a higher mortality rate [7, 10,11,12].
In recent years, endovascular interventional therapy has been developed and has gradually become the first choice in the treatment of SAA because it offers the advantages of minimal invasiveness, high success rate, fewer complications, rapid postoperative recovery, and maximal preservation of splenic blood supply.
Endovascular treatments include simple embolization of the aneurysmal sac, endovascular exclusion of splenic aneurysms with stent implantation, the sandwich technique by occlusion of the proximal and distal vessel with or without sac packing. In some cases, especially in giant SAA or in patients with comorbidities, a combination of several treatment techniques may be required, such as stent-assisted coil embolization. [7]
Occlusive agents such as coils, n-BCA or Onyx may be considered. Coils are the most widely used material and are available in a variety of sizes and shapes, from simple conical and cylindrical shapes to complex structures designed for specific applications [13].
Exclusion of the SAA with preserving the parent artery is possible by simple coiling or placement of a stent.
In simple coiling (sac-packing), the coils are placed in an aneurysmal sac until it is obliterated or excluded from the circulation. It was associated with the risk of coil migration, which has also been described in the treatment of renal artery aneurysms.
This technique is well suited for saccular aneurysms with a narrow neck, as the coils remain in the sac and the flow of the main vessel to the visceral organ is maintained, with a low risk of coil dislocation.
Covered stent placement is another option used to maintain native circulation.
The advantage of using a covered stent is that it creates a new lumen along the splenic artery and excludes the vascular lesion. The limiting factor of this approach is the tortuosity of the splenic artery, which can prevent advancement of the guiding catheter or sheath to an appropriate level of deployment.
Embolization of the single feeding artery would be unsatisfactory as branches of the pancreatic, gastric or distal arteries could act as retrograde collateral filling vessels and keep the aneurysm under pressure.
The aim is to achieve complete occlusion of the vessel beyond the aneurysmal sac to prevent rebleeding into the lesions through gastroepiploic, pancreatic or gastric collaterals.
This is the so-called sandwich technique, which involves distal and proximal embolization across the aneurysm sac. The “back door”, also known as the efferent artery, is usually closed first, followed by the “front door”, namely the afferent artery.
The sandwich technique allows complete exclusion of an aneurysmal sac as it prevents collateral circulation leading to persistent pressure on the sac, and it can also be used in complex configurations with multiple afferent or efferent vessels.
It is technically successful and has a very low complication rate, with only a small degree of splenic infarction. Perfusion of the spleen is maintained by collateral flow, primarily through the short gastric arteries.
Conclusions
SAAs are a rare clinical entity. Rupture is the most devastating complication and is associated with high morbidity and mortality.
Treatment is indicated when there is a high risk of bleeding, such as larger aneurysm (> 2 cm) or a symptomatic aneurysm.
Endovascular treatments are the first choice.The “sandwich technique” is a safe and effective treatment as it prevents bleeding back into the lesions through the collateral vessels.
Availability of data and material
Data concerning this case are available and easily accessible.
References
Kaya M, Baran Ş, Güya C, Kaplan MA (2016) Prevalence and predictive factors for development of splenic artery aneurysms in cirrhosis. Indian J Gastroenterol. https://doi.org/10.1007/s12664-016-0670-z
Veluppillai C, Perreve S, de Kerviler B, Ducarme G (2015) Splenic arterial aneurysm and pregnancy: a review. Presse Med. https://doi.org/10.1016/j.lpm.2015.06.009
Tcbc-Rj RA, Ferreira MC, Ferreira DA, Ferreira AG, Ramos FO (2016) Splenic artery aneurysm. Rev Col Bras Cir 43(5):398–400. https://doi.org/10.1590/0100-69912016005005. (PMID: 27982336)
Madoff DC, Denys A, Wallace MJ, Murthy R, Gupta S, Pillsbury EP et al (2005) Splenic arterial interventions: anatomy, indications, technical considerations, and potential complications. Radiographics. https://doi.org/10.1148/rg.25si055504
Rodríguez-Cordero M, González-Quintela A, Díaz-Peromingo JA (2014) Splenic artery aneurysm presenting with abdominal discomfort and weight loss. Acta Clin Belg. https://doi.org/10.1179/0001551214Z.00000000080
Sawicki M, Marlicz W, Czapla N, Łokaj M, Skoczylas MM, Donotek M et al (2015) Massive upper gastrointestinal bleeding from a splenic artery pseudoaneurysm caused by a penetrating gastric ulcer: case report and review of literature. Pol J Radiol. https://doi.org/10.12659/PJR.894465
Akbulut S, Otan E (2015) Management of giant splenic artery aneurysm: comprehensive literature review. Medicine. https://doi.org/10.1097/MD.0000000000001016
Manian U, Badri H, Coyne P, Nice C, Ashour H, Bhattacharya V (2009) Endovascular treatment of a ruptured splenic artery aneurysm using amplatzer R vascular plug. Int J Biomed Sci. https://doi.org/10.59566/IJBS.2009.5081
Al-Habbal Y, Christophi C, Muralidharan V (2010) Aneurysms of the splenic artery - a review. Surgeon. https://doi.org/10.1016/j.surge.2009.11.011
Wang CX, Han LN, Liang FQ, Chu FT, Jia X (2015) Aneurysm resection and vascular reconstruction for true aneurysm at the initial segment of splenic artery. J Huazhong Univ Sci Technolog Med Sci. https://doi.org/10.1007/s11596-015-1450-1
Shukla AJ, Eid R, Fish L, Avgerinos E, Marone L, Makaroun M et al (2015) Contemporary outcomes of intact and ruptured visceral artery aneurysms. J Vasc Surg. https://doi.org/10.1016/j.jvs.2015.01.005
Batagini NC, El-Arousy H, Clair DG, Kirksey L (2016) Open versus endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Ann Vasc Surg. https://doi.org/10.1016/j.avsg.2016.01.035
Jesinger RA, Thoreson AA, Lamba R (2013) Abdominal and pelvic aneurysms and pseudoaneurysms: imaging review with clinical, radiologic, and treatment correlation. Radiographics. https://doi.org/10.1148/rg.333115036
Acknowledgements
Thanks to OL, VA, VA, VR, VD, OD, TC
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Conception and design: VC, TL, SS, DR, FF. Writing the article: VC, TL, SS, DR, FF. Critical revision of the article: TL, SS, DR, FF. Final approval of the article: VC, TL, SS, DR, FF. Overall responsibility: FF.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Ethical approval and consent to participate
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Consent for publication
Consent obtained directly from patient.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Verde, C., Tarotto, L., Stilo, S. et al. Embolization of a splenic artery aneurysm: a case report. J Med Imaging Intervent Radiol 11, 8 (2024). https://doi.org/10.1007/s44326-024-00013-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44326-024-00013-2