Abstract
Pseudomyxoma peritonei (PMP) is an uncommon clinical condition, characterized by mucinous ascites, generally associated with a rupture of an epithelial neoplasm of the appendix. Some authors also use the term PMP to indicate mucinous dissemination after rupture of mucin-producing tumors of other sites (i.e. colon, ovary). Clinical presentation is variable and depends on the progressive accumulation of mucinous ascites (obstructive symptoms); weight loss, elevated Ca 19.9 and Ca 125 levels can be present. Here, we report a case of a 58-year-old patient with a history of appendicular mucocele and HCV-related hepatopathy, who accessed to the emergency department of our institute for the onset of epigastric pain associated with food vomiting for 5 days. Blood tests demonstrated a mild leukocytosis, a mild anemia (Hb 12.8 g/dL), and increased transaminases and cholestasis indices. A contrast-enhanced CT (CECT) scan was performed: a peritoneal cavity filled with a neoformation with lobulated margins and over-water density leading to multiple incisions of the hepatic and splenic parenchyma was documented. Appendix had an increased caliber (4 cm) with coprolite near its origin. Hence, the suspicion of PMP was raised and later confirmed on surgical exploration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pseudomyxoma peritonei (PMP) is a clinical syndrome characterized by progressive accumulation of mucinous tumor throughout the peritoneal cavity [1, 2]. The median age of patients affected by PMP is about 53 years with a predilection in females (2-3 more frequent); the incidence is approximately 0.2 per 100.000 population per year and develops in about 20% of patients with mucinous appendiceal neoplasms (MANs), but in some cases, it can also be present in patient affected by non-mucinous appendiceal adenocarcinoma (i.e. colon, ovary, and gallbladder) [3]. PMP has an indolent behavior, with a long natural history, and it grows undisturbed until the slow accumulation of mucin within the abdominal cavity leading to dyspnea or bowel obstruction, till eventually, death [4]. Elevated Ca 19.9 and Ca 125 levels can also be present [5, 6]. PMP has in general a poor prognosis, that can be improved to a 5-year overall survival (23–82%) thanks to an integrative therapeutic scheme, termed cytoreductive surgery—hyperthermic intraperitoneal chemotherapy (CRS-HIPEC) [7].
Case presentation
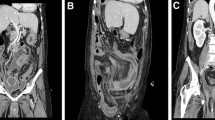
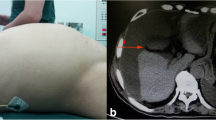
A 58-year-old man was referred to the emergency department for the onset of epigastric pain associated with vomiting intermingled with blood and inappetence in the past 5 days. The patient had in his medical history a chronic HCV-related hepatopathy, treated with antivirals (Glecaprevir and Pibrentasvir), type 2 diabetes mellitus, drugs addiction and appendicular mucocele. Blood tests performed demonstrated a mild leukocytosis (12.20 × 10^9/L), a mild anemia (Hb 12.8 g/dL), transaminase and cholestatic index values at upper limits. The emergency department clinician, therefore, requested an ultrasound (US) of the abdomen; given the patient's unexplorability due to intense intestinal meteorism and the patient's poor clinical condition, further investigation with contrast-enhanced CT (CECT) was immediately required. CECT examination documented an increased size of the liver, with finely irregular margins due to the known sclerogenic liver disease, and a transient hepatic attenuation difference (THAD) in the II hepatic segment. Moreover, CECT showed a voluminous neoformation with lobulated margins and an overwatered density occupying all supra-mesocolic and sub-mesocolic recesses leading to multiple incisions of liver and the spleen. A conspicuous layer of ascitic effusion was also documented.
The appendix showed increased caliber (about 4 cm) with a coprolite near its origin (Fig. 1a,b).
The image findings supported the hypothesis of PMP. In consideration of CECT findings and patient's medical history, further investigations were made. The oncologic markers Ca 19.9 and Ca 125 were indeed increased, respectively, 745 U/mL (range 0.0–39.0 U/mL) and 196 U/mL (range 0.0–35.0 U/mL). An exploratory laparoscopy with biopsy sampling of two peritoneal nodules and peritoneal fluid was performed. At the exploration, the peritoneal cavity was occupied by abundant ascitic fluid mixed with mucinous micelles. There was massive parietal and visceral peritoneal involvement of all abdominal quadrants; the involved loops of small bowel were agglomerated in the center and indissociable from the neoformation. Histological examination confirmed the presence of high-grade mucinous carcinoma peritonei. Because of the multiple comorbidities, the patient did not undergo to a cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC). However, systemic chemotherapy was started with oxaliplatin plus 5-fluorouracil scheme. About 18 months later, the patient died.
Discussion
PMP is a rare and poorly understood disease characterized by disseminated mucinous ascites and peritoneal, serosal, and omental mucinous implants [8]. As in most reports described in the literature, also in our case, a mucinous neoplasm of the appendix (MAN) was the trigger for the onset of PMP [9]. PMP is difficult to diagnose clinically; hence, imaging, particularly CECT, is crucial for a correct diagnosis and to assess the extent of the disease. On CECT typical findings are the presence of low attenuation masses (< 20 HU) scattered throughout the peritoneum with central displacement of the bowel loops. Other typical findings are prominent implants that cause a massive effect on the liver and the spleen producing the typical “scalloping” effect; the diaphragm can be thickened due to the implants of PMP in this site. Implants can present curvilinear peripheral calcifications. Sometimes PMP can metastasize to the ovary, so nodules at that level should not necessarily be considered as primary lesion. PMP only rarely tends to spread to the lymph nodes or directly to the thoracic cavity [6]. All these findings except linear calcifications were in line with our case. Over time, there have been various classification systems aimed to histologically characterize both the appendicular neoplasm, if present, and the degree of cellular atypia found in PMP. In 2016, the Peritoneal Surface Oncology Group International (PSOGI) developed a classification system aimed at grading epithelial neoplasms of the appendix [10]. The nomenclature is as follows:
-
o
Low-grade appendiceal mucinous neoplasm (LAMN) extends beyond the mucosa into the appendiceal wall, without infiltrative invasion; it has a low-grade cytologic atypia.
-
o
High-grade appendiceal mucinous neoplasm (HAMN) extends beyond the mucosa into the appendiceal wall, without infiltrative invasion; it has a high-grade cytologic atypia.
-
o
Mucinous adenocarcinoma shows an infiltrative invasion; it can be well, moderately, or poorly differentiated.
-
o
Poorly differentiated mucinous adenocarcinoma with signet ring features.
Although this classification system has a role in the prognostic definition of MANs and the likelihood of developing PMP is lower in poorly differentiated forms, the PSOGI system recommends that PMP be histologically classified based on peritoneal pathology rather than primary neoplasm. The nomenclature is as follows:
-
o
Acellular mucin: absence of neoplastic epithelial cells within the peritoneal mucin.
-
o
Low-grade mucinous carcinoma peritonei—G1, previously referred to as disseminated peritoneal adenomucinosis (DPAM).
-
o
High-grade mucinous carcinoma peritonei—G2, previously referred to as peritoneal mucinous carcinomatosis (PMCA).
-
o
High-grade mucinous carcinoma peritonei with signet ring cells—G3, previously referred to peritoneal mucinous carcinomatosis with signet ring cells (PMCA - S) [10].
Cytoreductive surgery (CRS) in combination with (HIPEC) is the foundations of treatment of PMP [11]. Cytoreduction combines multiple peritonectomies—parietal, diaphragmatic and pelvic—and visceral resections—gastrointestinal, hysterectomy and splenectomy—with the “electro evaporation” of unresectable nodules [12]; the goal is to achieve a complete cytoreduction in the absence of macroscopically visible lesions. HIPEC is performed after the completion of surgery; most centers use Oxaliplatin–Mitomycin C chemotherapy in a temperature range of 41.5–43 °C with a perfusion time from 30 to 90 min [12]. Being a highly demolitive treatment, preliminary evaluation (either laparoscopic or CECT) of the extent of the disease is necessary. In this regard, in 1995, Sugarbaker quantified the dispersion of abdominal disease through numerical values correlated to quadrants of the abdomen, determining the peritoneal carcinomatosis index (PCI) [13]. The cytoreductive surgery (CCP) can be classified with the following score:
-
o
CC0: no residual tumor.
-
o
CC1: residual tumor < 2.5 mm.
-
o
CC2: residual tumor 2.6 mm to 2.5 cm.
-
o
CC3: residual tumor > 2.5 cm.
The aim of CCP is to achieve a CC0–CC1 score, which can be considered a complete cytoreduction that gives the patient a good prognosis compared with patients with a CC2–CC3 [14]. An intraoperative PCI > 20 is considered a large disease burden and a relative contraindication to CRS as the potential risks are thought to outweigh benefits [15], although some studies affirm that even in the presence of a PCI > 20, low-grade lesions can still be treated with CRS-HIPEC with an acceptable long-term outcome [16]. These observations are in line with the reported case, which indeed had a PCI > 20, a high-grade appendiceal lesion, and multiple comorbidities (diabetes mellitus, HCV-related chronic hepatopathy, and drug addiction). Therefore, our patient was initiated on palliative systemic chemotherapy with oxaliplatin plus 5-fluorouracil scheme. Many studies have documented a fair degree of chemoresistance, either due to the low degree of cell proliferation or the mucin-rich microenvironment that limits the availability of the drugs [17], and thus a worse prognosis than patients who can benefit from CRS-HIPEC.
Conclusion
PMP is a rare disease generally associated to MANs. On CECT, the suspicion of PMP can be proposed if there are clinical and anamnestic data to support the diagnosis. HIPEC-CRS is the gold standard of treatment; but in view its rarity and prognosis, a multidisciplinary approach is always necessary to ensure the best therapeutic outcome.
Data availability
The data presented in this study are available on request from the corresponding author.
References
Moran BJ, Cecil TD (2003) The etiology, clinical presentation, and management of pseudomyxoma peritonei. Surg Oncol Clin N Am 12(3):585–603. https://doi.org/10.1016/s1055-3207(03)00026-7. (PMID: 14567019)
Carr NJ, Bibeau F, Bradley RF, Dartigues P, Feakins RM, Geisinger KR, Gui X, Isaac S, Milione M, Misdraji J, Pai RK, Rodriguez-Justo M, Sobin LH, van Velthuysen MF, Yantiss RK (2017) The histopathological classification, diagnosis and differential diagnosis of mucinous appendiceal neoplasms, appendiceal adenocarcinomas and pseudomyxoma peritonei. Histopathology 71(6):847–858. https://doi.org/10.1111/his.13324. (Epub 2017 Sep 19 PMID: 28746986)
Smeenk RM, van Velthuysen ML, Verwaal VJ, Zoetmulder FA (2008) Appendiceal neoplasms and pseudomyxoma peritonei: a population based study. Eur J Surg Oncol 34(2):196–201. https://doi.org/10.1016/j.ejso.2007.04.002. (Epub 2007 May 23 PMID: 17524597)
Santullo F, Pacelli F, Abatini C, Attalla El Halabieh M, Fortunato G, Lodoli C, Giovinazzo F, Rotolo S, Di Giorgio A (2021) Cytoreduction and hyperthermic intraperitoneal chemotherapy for pseudomyxoma peritonei of appendiceal origin: a single center experience. Front Surg 8:715119. https://doi.org/10.3389/fsurg.2021.715119. (PMID: 34513915; PMCID: PMC8427490)
Zaheer A, Raman S et al (2022) Diagnostic imaging - gastrointestinal. Elsevier
Bartlett DJ, Thacker PG Jr, Grotz TE, Graham RP, Fletcher JG, VanBuren WM, Iyer VR, Fidler JL, Menias CO, Wasif N, Sheedy SP (2019) Mucinous appendiceal neoplasms: classification, imaging, and HIPEC. Abdom Radiol (NY) 44(5):1686–1702. https://doi.org/10.1007/s00261-018-01888-y. (PMID: 30610247)
Rizvi SA, Syed W, Shergill R (2018) Approach to pseudomyxoma peritonei. World J Gastrointest Surg 10(5):49–56. https://doi.org/10.4240/wjgs.v10.i5.49. (PMID:30190782;PMCID:PMC6121001)
Li C, Kanthan R, Kanthan SC (2006) Pseudomyxoma peritonei–a revisit: report of 2 cases and literature review. World J Surg Oncol 1(4):60. https://doi.org/10.1186/1477-7819-4-60. (PMID:16945158;PMCID:PMC1574320)
García KM, Flores KM, Ruiz A, González FL, Rodríguez ÁM (2019) Pseudomyxoma peritonei: case report and literature review. J Gastrointest Cancer 50(4):1037–1042. https://doi.org/10.1007/s12029-018-00192-8. (PMID: 30618002)
Carr NJ, Cecil TD, Mohamed F, Sobin LH, Sugarbaker PH, González-Moreno S, Taflampas P, Chapman S, Moran BJ, Peritoneal Surface Oncology Group International (2016) A consensus for classification and pathologic reporting of pseudomyxoma peritonei and associated appendiceal neoplasia: the results of the Peritoneal Surface Oncology Group International (PSOGI) modified delphi process. Am J Surg Pathol 40(1):14–26. https://doi.org/10.1097/PAS.0000000000000535. (PMID: 26492181)
Wahba R, Schmidt T, Buchner D, Wagner T, Bruns CJ (2023) Chirurgische therapie des pseudomyxoma-peritonei-syndroms – zytoreduktive chirurgie und hypertherme intraperitoneale chemotherapie [surgical treatment of pseudomyxoma peritonei-cytoreductive surgery and hyperthermic intraperitoneal chemotherapy]. Chirurgie (Heidelb) 94(10):840–844. https://doi.org/10.1007/s00104-023-01937-3. (Epub 2023 Aug 14. PMID: 37578542)
Sommariva A, Tonello M, Rigotto G, Lazzari N, Pilati P, Calabrò ML (2021) Novel perspectives in pseudomyxoma peritonei treatment. Cancers (Basel) 13(23):5965. https://doi.org/10.3390/cancers13235965. (PMID:34885075;PMCID:PMC8656832)
Jacquet P, Sugarbaker PH (1996) Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 82:359–74. https://doi.org/10.1007/978-1-4613-1247-5_23. (PMID: 8849962)
Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, Baratti D, Deraco M, Elias D, Sardi A, Liauw W, Yan TD, Barrios P, Gómez Portilla A, de Hingh IH, Ceelen WP, Pelz JO, Piso P, González-Moreno S, Van Der Speeten K, Morris DL (2012) Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol 30(20):2449–56. https://doi.org/10.1200/JCO.2011.39.7166. (Epub 2012 May 21 PMID: 22614976)
Grotz TE, Overman MJ, Eng C, Raghav KP, Royal RE, Mansfield PF, Mann GN, Robinson KA, Beaty KA, Rafeeq S, Matamoros A, Taggart MW, Fournier KF (2017) Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for moderately and poorly differentiated appendiceal adenocarcinoma: survival outcomes and patient selection. Ann Surg Oncol 24(9):2646–2654. https://doi.org/10.1245/s10434-017-5938-8. (Epub 2017 Jul 10 PMID: 28695394)
Jimenez W, Sardi A, Nieroda C, Sittig M, Milovanov V, Nunez M, Aydin N, Gushchin V (2014) Predictive and prognostic survival factors in peritoneal carcinomatosis from appendiceal cancer after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 21(13):4218–25. https://doi.org/10.1245/s10434-014-3869-1. (Epub 2014 Jul 2 PMID: 24986239)
Asare EA, Compton CC, Hanna NN, Kosinski LA, Washington MK, Kakar S, Weiser MR, Overman MJ (2016) The impact of stage, grade, and mucinous histology on the efficacy of systemic chemotherapy in adenocarcinomas of the appendix: analysis of the National Cancer Data Base. Cancer 122(2):213–21. https://doi.org/10.1002/cncr.29744. (Epub 2015 Oct 27. PMID: 26506400; PMCID: PMC4860278)
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Matilde Anichini, Antonella Masserelli and Lavinia Mattolini: conception, acquisition of data, literature search, interpretation of data and writing of the manuscript. Giulia Grazzini and Vittorio Miele conception: interpretation of data, supervision, and writing of the manuscript. All the authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards (31/11/2021 CEAVC 21101).
Informed consent
Informed consent was obtained from all subjects involved in the study.
Consent for publication
The authors affirm that human research participants provided informed consent for publication.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Anichini, M., Grazzini, G., Masserelli, A. et al. Peritoneal pseudomyxoma in a patient affected by appendicular mucocele: a case report. J Med Imaging Intervent Radiol 11, 16 (2024). https://doi.org/10.1007/s44326-024-00012-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44326-024-00012-3