Abstract
Seaweeds are grown for their use in food and other sectors, however heavy metals (HMs) contamination raises serious issues for the environment and public health. This study focuses on seaweed samples (Hypnea musciformes and Gracilaria lemaneiformis) collected from the Rezu Khal seaweed culture site, Cox’s Bazar coast, Bangladesh. HMs and minerals were determined using Atomic Absorption Spectrophotometry (AAS). Among the elements examined, H. musciformes displayed the highest mean concentrations of minerals and HMs, including Magnesium (Mg) (8663.00 ± 2302.06 mg/kg), Copper (Cu) (10.59 ± 1.61 mg/kg), Iron (Fe) (7566.29 ± 2842.47 mg/kg), Manganese (Mn) (9.93 ± 2.88 mg/kg), Zinc (Zn) (29.54 ± 7.51 mg/kg), and Nickel (Ni) (11.77 ± 2.63 mg/kg). Conversely, G. lemaneiformis exhibited the highest levels of Calcium (Ca) (798.14 ± 143.40 mg/kg), Lead (Pb) (3.91 ± 1.74 mg/kg), and Chromium (Cr) (0.59 ± 0.30 mg/kg). Specifically, Mg was prominently abundant in H. musciformes, while Cawas more prevalent in G. lemaneiformis. Both seaweed types contained Pb and Cr. None of the elements was significantly different between the species (p < 0.05). The consumers are not exposed to any carcinogenic or non-carcinogenic risks related to these concentrations, according to the evaluation of carcinogenic and non-carcinogenic risks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Seaweeds are creatures that resemble plants and grow along the shore. They use holdfasts to cling to the sea floor or any other hard substrate [1,2,3,4]. Seaweeds also have an ecological role in reducing climate change impacts [5,6,7]. Since the dawn of human civilization, seaweeds have played a crucial role in the diets of Korea, Japan, China, and some Southeast Asian countries, either directly or through other food groups [8, 9]. Since seaweed has high medicinal and nutritional benefits, they are currently in high demand in the international market. Seaweed contains vitamins, minerals, proteins, flavonoids, and soluble fiber, which are considered preventive medicines against diseases caused by a sedentary lifestyle [10]. Recent extensive cohort studies in Japan have shown that seaweed consumption reduces the risk of cardiovascular disease [10]. Therefore, seaweed aquaculture has become one of the blue economic measures around the world, and it is practiced in several countries such as China, Indonesia, Korea, the Philippines, Japan, and Malaysia [11]. Seaweed aquaculture is now a very small business in Bangladesh, mostly restricted to the southeast coastal region, particularly in certain areas of Cox's Bazar [12]. Bangladesh has about 8500 km2 shallow (< 5 m depth) nearshore and offshore areas where 335 varied species of marine seaweeds occur naturally. Some of these seaweeds are cultured on a small scale due to socio-economic, technological, and environmental constraints [12].
Seaweed is regarded as one of the most significant marine resources for Bangladesh's achievement of its blue economy aim [13, 14]. To advance the industry, the government has added seaweed culture in the 7th Five-Year Plan and has launched seaweed farming initiatives through different organizations [12]. In Bangladesh's coastal and marine habitats, about 32 species of the 335 recorded seaweed species are abundant [12, 15]. Due to their growth ability and accessibility of wild seeds, 14 of these species (10 Rodophyta species and 4 Chlorophyta species) are suitable for cultivation [15]. Many species of the genera Gracilaria, Hypnea, and Ulva can grow in brackish water ecosystems such as the mouth of the Bakhkhali River and the Moheshkhali and Kutubdia canals, although most seaweeds live in marine ecosystems. Species of the genera Gracilaria, Hypnea, Kappaphycus, and Porphyra, in particular, have high commercial value because they are used as a source for the production of agar and carrageenan and as vegetables for human consumption [12].
At present days, HMs in aquatic environments has been a global concern [16,17,18]. Along with this, concerns regarding potential HMs pollution in seaweeds have recently been voiced [19]. The marine ecosystem eventually receives human-caused metals released from mineral extraction, petrochemicals, printing, electronic, and urban waste sources [20,21,22]. When harmful metals find their way into aquatic environments where seaweed grows, these plants can eventually uptake these metals, which can be transferred to the human body through the food web [23,24,25,26]. Certain biologically necessary elements may have harmful effects at higher concentrations, and some metals, like Cd, Hg, and Pb, can be hazardous even in trace amounts [27]. The human body's internal organs and fatty tissues can store HMs, which can have an impact on the central nervous system [28]. Notably, the metalloid element arsenic exhibits a variety of forms and toxicities [29]. Compared to organic arsenic, which is genotoxic, and a recognized human carcinogen particularly linked to skin, lung, bladder, and liver cancer, inorganic arsenic exhibits far greater lethality [30,31,32,33]. However, there is a concerning lack of research on the accumulation of minerals and HMs in seaweed along the Bay of Bengal (BoB), Bangladesh. To assess the suitability of seaweeds for consumption or industrial applications, understanding the levels of essential minerals and potentially harmful HMs in seaweed species is necessary. Therefore, the main objective of this study was to conduct an inclusive assessment of the minerals (Ca and Mg) and HMs (Cu, Fe, Mn, Zn, Ni, Pb, and Cr) concentrations within two specific species of red seaweed, H. musciformes, and G. lemaneiformis. These seaweeds were collected from the Rezu Khal region in the BoB in Bangladesh. The investigation focused on analyzing and quantifying the levels of essential minerals and potentially harmful HMs in these seaweed species. By addressing this objective, the research sought to provide valuable insights into the nutritional composition and potential contaminants in these seaweeds, which are crucial for understanding their ecological role and assessing their suitability for consumption or industrial applications. Additionally, the goal of this investigation was to advance understanding of the health and environmental effects of seaweed use and farming in this area.
2 Materials and methods
2.1 Study area
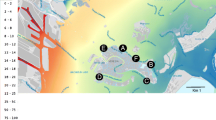
Rezu Khal on the coast of Cox's Bazar was selected for cultivation after a feasibility analysis (Fig. 1). This culture region was quite close to the coast, where salinity ranges from 23 to 30 ppt.
2.2 Geographical information
Rezu Khal is a topographical feature in the Bangladeshi district of Cox's Bazar. Cox's Bazar is situated in the southeastern part of the country, along the BoB. Rezu Khal is a stream or canal that flows through the region, contributing to the overall landscape and hydrology of Cox's Bazar. The location of the seaweed farm is near the sea. As a result, saline water enters the khal. There are many mangrove trees near the sites. Chemically altered or eutrophic water is frequently discharged into the Khal from a tilapia farming facility (Niribili Monosex Tilapia Hatchery) to the west. The soil at the breeding site is mainly sandy, while the bank of the Khal is mainly muddy. As a result, there is tremendous sedimentation, and the water is not very transparent. Both at low tide and high tide, the water flows strongly. As a tidal creek, Rezu Khal experiences the ebb and flow of tides, which influences its water levels and flow patterns. During high tide, the water in Rezu Khal rises, while during low tide, the water recedes, exposing the mudflats and revealing the channel's distinct characteristics. It experiences a monsoon climate characterized by heavy rainfall. The region receives substantial precipitation during the monsoon season, typically from June to September. The heavy rains contribute to the overall water volume and flow in Rezu Khal, affecting its depth and velocity. The seasonal changes also influence Rezu Khal. In the dry season, which spans from October to May, the water levels in the creek tend to be lower, and the flow may be reduced. During this time, the stream may have slower currents and narrower channels. On the other hand, Rezu Khal experiences faster flow and higher water levels during the wet season because of increased rainfall.
2.3 Determination of minerals and heavy metals in seaweeds
To prevent contamination, the samples were handled with extreme caution. Glassware was thoroughly cleaned using distilled water and chromic acid. Throughout the investigation, analytical-grade materials and reagents were employed. Instrument measurements were adjusted using reagent blank determinations. Below is a brief description of the methods used for sample preparation, standard preparation, and analysis for the metal analyses.
2.4 Sample collection and preparation
During low tide, seaweeds were collected in December, January, and February 2023–24. The winter season was chosen because there is typically less rainfall, which can help reduce the washout of metals from terrestrial sources into the coastal waters. This can result in lower concentrations of metals in seaweed samples, providing a more precise representation of the metal levels. The collected seaweeds were transported back to the laboratory in insulated containers. Upon arrival, they were thoroughly washed in multiple changes of fresh water and rinsed with distilled water to remove any epiphytes and herbivores. Subsequently, the samples were pre-frozen at − 40 °C and then freeze-dried until the moisture content reached below 10%. Three replicate samples were gathered for the analysis of the two seaweed species from the study area. In cases where there was insufficient material for three analyses during a single collection, the triplicate samples were compiled using material collected on different sampling dates. A total of 14 seaweed samples (7 H. musciformes and 7 G. lemaneiformis) were collected from the study area.
After that, organic material was likewise destroyed. It was necessary to exercise caution to prevent losses from elemental volatilization. The materials were precisely weighed (10–20 g) and placed in a tared silica dish. After that, the samples were dried in a lab oven at 120 °C. After that, the dishes were put in the muffle furnace at room temperature and gradually heated to 450 °C at a rate that would not go above 50 °C per h. The samples were burned at 450 °C in a muffle furnace for a minimum of 8 h. Trays were taken out of the furnace once the samples had cooled. After that, the sediment samples were cooked on a hot plate in the appropriate volume of 50% nitric acid. After that, the samples were filtered using Whatman No. 44 filter paper into a 100 ml volumetric flask, and the leftover material was cleaned. Distilled water was used to top off each sample solution.
2.5 Standard preparation
The metal standard solutions could degrade over time; thus, each one was produced to calibrate the instrument for each element to be determined on the same day that the analyses were conducted. Distilled water and chemicals of the highest caliber were used to create each sample. One gram each of the metals Cr, Cu, Pb, and Ni was dissolved in HNO3 solution; 1 g each of Ca, Mg, Fe, Mn, and Zn was dissolved in HCl solution; 2.8289 g of K2Cr2O7 (= one gram of Cr) was dissolved in water and filled a volumetric flask to one liter with distilled water to create a stock solution of 1000 mg/l of Cd, Cu, Pb, Ni, Co, Fe, Mn, Zn, Al, and Cr. Next, using micropipettes in 5 ml of 2N HNO3, 100 ml of the working standards of every metal aside from Fe were created at concentrations of 0.1, 0.25, 0.5, 0.75, 1.0, and 2.0 mg/l. The iron stock solution was used to prepare 100 ml of the working standards for Fe (2.0, 2.5, 5.0, 10.0, and 20.0 mg/l). To prevent reagent contamination, the reagent blank was made in the same manner as the sample preparation without the sample.
2.6 Analytical measurement
Atomic Absorption Spectrophotometry (AAS, iCE 3300, Thermo Scientific, made in China) used standard analytical techniques to quantify the HMs concentrations of the collected seaweeds (Table 1). The manufacturer's recommended operational parameters and matrix modifiers were utilized during the analysis. The analyte's optimal absorption and flame conditions were achieved by setting up the atomic absorption equipment. After that, the AAS flame was aspirated with blanks (deionized water), standards, sample blanks, and samples. Plotting calibration curves for concentration vs absorbance was done. The least squares method was used to fit a straight line to the data to do statistical analysis. In addition, a blank measurement was taken, and any required adjustments were applied when determining each element's concentration. The detection limits (mg/kg), expressed in mg/kg dry weight, were as follows: Pb: 0.008, Cu: 0.2, Zn: 0.002, Mn: 0.011, Ni: 0.004, Cr: 0.072, Ca: 0.1, Mg: 0.01 and Fe: 0.01.
2.7 Assessment of human health risk from seaweed consumption
2.7.1 Estimated daily intake (EDI)
The determination of the EDI is crucial to assess the possible health risks related to the intake of aquatic food products contaminated with metals [31]. In this scenario, the EDI can be evaluated by considering the metal concentrations present in aquatic food items and their regular consumption [34]. The estimation of the EDI can be accomplished by employing the equation provided by the USEPA, as outlined in previous studies [35, 36]. This rigorous approach ensures the calculation is conducted with utmost care and attention, guaranteeing a plagiarism-free analysis.
The metal concentration of the metal contents (expressed in mg/kg-dry weight) is denoted as CN, while the ingestion rate is represented by IGr. The FAOSTAT database (2012 period) provides the specific ingestion rates for adults (55.5 g/day) and children (52.5 g/day) [31]. The body weight of the local consumers is indicated as BWt, with adults having a weight of 70 kg and children weighing 15 kg [37].
2.7.2 Non-carcinogenic risk assessment
The Target Hazard Quotient (THQ) estimation is conducted to estimate the probable risks linked with exposure to metal contaminants resulting from consuming contaminated food items [38]. In this context, the THQ can be determined by calculating the ratio between the EDI and the oral reference dose (RfD). The RfDs for specific metal contaminants, namely Cu, Fe, Mn, Zn, Ni, Pb, Cr, and Ca, are 4.0E−02, 7.0E−01, 1.4E−01, 3.0E−01, 1.1E−02, 2.00E−03, 3.0E−03 and 1.0E−03, respectively [37]. RfD of Mg is not available; hence, health risk was assessed excluding Mg concentration. The calculation of the THQ adheres to the equation established by Heshmati et al. [39] and Ahmed et al. [32]:
The variable Ed represents the duration of exposure to the metal contents, which is set at 65 years based on the information provided by USEPA [37]. Ep denotes the frequency of exposure, assumed to be 365 days per year, according to Ahmed et al. [40]. The mean time for the non-carcinogenic elements, At, is determined by multiplying Ed by Ep. If At yields value less than 1, it indicates that there are no non-carcinogenic effects on the local consumers, as established by Abtahi et al. [41].
2.7.3 Hazard index (HI) evaluation
The HI assessment is crucial in determining the possible risks connected with humans' consumption of various contaminated food items. It is essential to consider that exposure to multiple contaminants can lead to additive and interactive effects on human health, as Saha et al. [42] highlighted. The HI for these multiple contaminants can be evaluated following the equation provided by Ahmed et al. [31, 32] and Zhao et al. [43].
In this context, THQ represents the risk assessment value associated with the presence of multiple metal contents in the samples. If the THQ value exceeds 1, it indicates the presence of significant non-carcinogenic impacts on human health, as stated by Hossain et al. [44]. These rigorous measurements and references ensure a better understanding of THQ and its implications in evaluating the potential health effects resulting from exposure to multiple metal contaminants [25].
2.8 Statistical analysis
The mean and standard deviation of metal concentrations were calculated, and statistical tests were conducted using SPSS V.22 software. The Kolmogorov–Smirnov and Shapiro–Wilk tests were utilized to assess the normality test of the data [45]. To identify significant variations in the targeted elements among the specimens in the studied area, the ANOVA test was employed for each species with a significance level set at p < 0.05 (indicating a 95% confidence level). Additionally, Levene's test was applied to determine the homogeneity of variances for ANOVA tests, with a significance level of p < 0.05 [32, 45]. Experimental results were expressed using the mean ± SD of triplicate samples.
3 Results and discussion
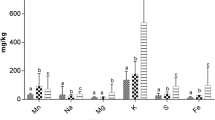
In the present study, the concentrations of two minerals and seven HMs were analyzed. The concentrations of available minerals and HMs in H. musciformes and G. lemaneiformis seaweeds are shown in Table 1, where no noteworthy difference (p > 0.05) was found between the two species. The highest mean amount of minerals and HMs, i.e., Mg (8663.00 ± 2302.06 mg/kg), Cu (10.59 ± 1.61 mg/kg), Fe (7566.29 ± 2842.47 mg/kg), Mn (9.93 ± 2.88 mg/kg), Zn (29.54 ± 7.51 mg/kg) and Ni (11.77 ± 2.63 mg/kg) was detected in H. musciformes. On the other hand, the highest volume of Ca (798.14 ± 143.40 mg/kg), Pb (3.91 ± 1.74 mg/kg), and Cr (0.59 ± 0.30 mg/kg) was reported in G. lemaneiformis. The statistical test revealed that the species' element concentrations significantly differed (p ≤ 0.05). Moreover, the calculated Kolmogorov–Smirnov and Shapiro–Wilk tests indicated that element concentration was non-normal distributed in the species. Furthermore, the calculated Levene's test determined evidence of significant heterogeneity in variances (p < 0.05).
3.1 Minerals
3.1.1 Calcium (Ca)
Calcium (Ca) is an essential mineral that is naturally present in seaweed and offers numerous health benefits to humans. Seaweed consumption can provide a valuable source of dietary Ca, which plays a crucial role in maintaining strong bones and teeth, promoting proper muscle function, and aiding in nerve transmission. Ca is linked to healthy bones and teeth, but it is also essential for blood coagulation, contraction of muscles, brain activity regulation, and normal heartbeat. The bones contain 99% of the Ca that the human body has; the remaining 1% is found in the blood, muscles, and other tissues. Adequate Ca intake from seaweed can help prevent osteoporosis and contribute to overall bone health. However, it is important to note that excessive intake of Ca can lead to toxicity and adverse health effects. When Ca levels exceed the recommended limits, it can cause constipation, and kidney stones, and interfere with the absorption of other minerals, such as Fe and Zn. In this study, the highest mean Ca amount was recorded in G. lemaneiformis (798.14 ± 143.40 mg/kg) and the lowermost in H. musciformes (767.14 ± 183.81 mg/kg) (Table 1). Siddique et al. [8] studied seaweeds at Saint Martin's Island (SMI) in the BoB, Bangladesh, and found 496.26 ± 2.45 mg/kg and 484.18 ± 4.68 mg/kg Ca in H. pannosa and H. musciformis, respectively (Table 2).
3.1.2 Magnesium (Mg)
Magnesium (Mg) is an essential mineral found in seaweed that offers numerous health benefits to humans. Seaweed consumption can provide a valuable source of dietary Mg, which is involved in various physiological processes. Mg plays a vital role in maintaining proper nerve and muscle function, regulating blood pressure, supporting a healthy immune system, and promoting bone health. Adequate Mg intake from seaweed can also help alleviate symptoms of migraines, reduce the risk of cardiovascular diseases, and improve mood and mental well-being. Moreover, Mg aids in the creation of energy and other essential bodily processes, including the health of muscles and neurons. However, it is important to note that excessive intake of Mg can lead to toxicity and adverse health effects. When Mg levels exceed the recommended limits, it can cause gastrointestinal issues, diarrhea, and interfere with the absorption of other minerals, such as calcium and zinc. As shown in the data, Mg is the most abundant element in both seaweeds and the amounts ranged from 3600.00–10080.00 mg/kg and 5400.00–9600.00 mg/kg in H. musciformes and G. lemaneiformis, respectively (Table 1). Siddique et al. [8] found 3278.12 ± 28.04 mg/kg and 2112.70 ± 17.80 mg/kg Mg in H. pannosa and H. musciformis, respectively (Table 2).
3.2 Heavy metals
The mean values of the seven HMs amount for two algal species, namely H. musciformes and G. lemaneiformis, followed the same order Fe > Zn > Ni > Cu > Mn > Pb > Cr, and no substantial variation (p > 0.05) was detected between the two species. In contrast to G. lemaneiformis, significantly higher amounts of all HMs studied were found in H. musciformes, which can be seen in Table 1.
3.2.1 Copper (Cu)
The biochemistry of all living things requires the trace element Cu, which is essential for life [46]. Cu is a vital element as it plays a crucial role in the synthesis of hemoglobin and is present in several enzymes. Nonetheless, it is worth mentioning that excessive levels of copper can result in acute toxicity. However, in Bangladesh, there is no adopted legislation specifying the highest permissible amount of HMs in various edible seaweeds. The Australian and New Zealand Food Authority (2005) recommended a Cu content of 10 ppm in 240 fresh seaweeds as the maximum allowable limit [47]. The highest mean Cu concentration was found in H. musciformes (10.59 ± 1.61 mg/kg) and the lowermost in G. lemaneiformis (10.15 ± 1.05 mg/kg) (Table 1). Malea and Kevrekidis [48] recorded 3.29 mg/kg Cu in Gracilaria and 7.22 mg/kg in Hypnea from the Gulf of Thessaloniki, Aegean. Ali et al. [21] recorded a lower amount of Cu in Gracilaria and Hypnea from the Sudanese coast of the Red Sea (Table 2). Here, both seaweeds exceeded the permissible limits (10 mg/kg) for Cu concentrations in plant tissue [49].
3.2.2 Iron (Fe)
Iron (Fe) deficiency is a global problem, although it is one of the most abundant HMs in the Earth's crust [50]. Fe is an essential mineral that is found naturally in seaweed and offers numerous health benefits to humans. Seaweed, being a rich source of Fe, can contribute to maintaining healthy blood and preventing Fe deficiency anemia. Fe is a key component of hemoglobin, the protein responsible for transporting oxygen throughout the body. Consuming seaweed can help increase Fe levels, supporting optimal oxygen delivery, energy production, and overall cellular function. Furthermore, Fe plays a vital role in immune function, cognitive development, and maintaining healthy skin, hair, and nails. As shown in Table 1, Fe was the most abundant HM in the two red seaweeds studied (7566.29 ± 2842.47 mg/kg and 6038.57 ± 2361.28 mg/kg in H. musciformes and G. lemaneiformis, respectively). Red seaweeds are reported to contain more Fe than brown and green seaweeds [51]. Siddique et al. [8] recorded 621.66 mg/kg and 659.32 mg/kg Fe in H. pannosa and H. musciformis (Table 2).
3.2.3 Manganese (Mn)
Manganese (Mn) is a trace mineral that is naturally present in seaweed and offers several health benefits to humans. Seaweed consumption can provide a dietary source of Mn, which plays a crucial role in various enzymatic reactions and the production of antioxidant enzymes. Mn is involved in bone development, wound healing, and the metabolism of carbohydrates, proteins, and cholesterol. However, it is important to be aware that excessive intake of Mn can be toxic to human health. When Mn levels exceed the recommended limits, it can accumulate in the body and lead to neurological issues, such as Parkinson's-like symptoms, cognitive impairment, and behavioral changes. It is therefore essential to maintain a balanced intake of Mn from seaweed and other dietary sources, ensuring it remains within safe levels to prevent any potential toxicity. A high requirement for Mn was found in every species studied [52]. H. musciformes and G. lemaneiformis contained 9.93 ± 2.88 and 9.71 ± 3.68 mg/kg Mn, respectively. Siddique et al. [8] found a much higher Mn concentration in seaweeds at SMI in the BoB, Bangladesh. Malea and Kevrekidis [48] recorded 163.3 mg/kg in Gracilaria sp. and 61.78 mg/kg of Hypnea sp. from the Gulf of Thessaloniki, Aegean (Table 2).
3.2.4 Zinc (Zn)
Zinc (Zn) is a naturally produced HM that is found in all plants and animals. Zn is an essential mineral found in seaweed that offers numerous health benefits to humans. Seaweed consumption can provide a valuable source of Zn, which plays a vital role in various physiological processes, including immune function, wound healing, and DNA synthesis. It is supportive of normal development in humans, particularly in the early stages of life [53, 54]. Zn also maintains healthy skin, promotes proper growth and development, and supports reproductive health. However, maintaining an appropriate Zn intake balance is crucial, as excessive levels can lead to toxicity. When Zn levels exceed the recommended limits, it can interfere with the absorption of other essential minerals, such as Cu and Fe, and cause adverse effects on human health. Zn levels in fresh 264 seaweed should not exceed 14 ppm, according to the Australian and New Zealand Food Authority (2005). In the present study, the mean Zn concentrations in H. musciformes and G. lemaneiformis were 29.54 ± 7.51 and 29.30 ± 5.82 mg/kg, respectively. The recorded amount is higher than the amount recorded by Anbazhagan et al. [55], Abdallah [56], and Siddique et al. [8] (Table 2).
3.2.5 Nickel (Ni)
Nickel (Ni) is a trace element found in seaweed and has potential health benefits and toxicity concerns for humans. Seaweed consumption can provide a source of Ni, which is involved in various metabolic processes and enzyme functions. Ni is essential in the body for proper cell growth, immune function, and DNA repair. However, it is important to note that excessive intake of Ni can lead to toxicity and adverse health effects. When Ni levels exceed the recommended limits, it can cause some individuals allergic reactions, skin irritation, and respiratory issues. Long-term exposure to high levels of Ni has also been associated with an increased risk of certain cancers. In general, Ni can potentially affect the general population by ingesting contaminated food and water [57]. The mean Ni concentrations in H. musciformes and G. lemaneiformis were 11.77 ± 2.63 and 11.68 ± 3.72 mg/kg, respectively. The present study shows that Ni concentration in seaweeds in Rezu Khal is higher than in seaweeds from SMI, the Gulf of Thessaloniki, and the Sudanese Red Sea coast [8, 22, 48] (Table 2).
3.2.6 Lead (Pb)
Lead (Pb) is a HM that can be found in seaweed and poses significant health risks to humans. While seaweed is beneficial in many ways, it is important to note that Pb can accumulate in seaweed through environmental contamination. Consumption of seaweed contaminated with Pb can lead to severe health issues. Excessive intake of Pb can cause neurological problems, developmental delays in children, impaired cognitive function, and damage to vital organs like the kidneys and liver [58, 59]. Furthermore, Pb exposure has been linked to an increased risk of hypertension, cardiovascular disease, and reproductive disorders. It is crucial to ensure that seaweed and all food sources are regularly tested for lead contamination to protect human health and prevent any potential toxicity. Pb is a typical HM that poses a major risk to human health, especially in developing countries [60]. However, there is no information on regulatory restrictions on the amount of Pb in edible seaweeds [61]. The European Commission (EC, 2011) has recommended a Pb level of 3 ppm, and the maximum level recommended by France for seaweed is also 5 mg/kg dry weight [62]. Human body systems and organs are affected by Pb poisoning [63, 64]. Pb exposure, even at modest doses (3.0 g/kg/day), can negatively affect a person's IQ, learning ability, and attention span [63, 64]. Seaweed intake is generally thought to pose a low risk of Pb exposure to humans [65]. In the present study, Pb concentrations in the seaweed ranged from 2.22 mg/kg to 7.35 mg/kg (Table 1). The average concentration of Pb in H. musciformes is 3.56 mg/kg, while in G. lemaneiformis the concentration was recorded 3.91 mg/kg (Table 2).
3.2.7 Chromium (Cr)
Chromium (Cr) can occur in a variation of oxidation states from 0 to 6+. Trivalent Cr (III) is much less soluble in plants than hexavalent Cr (VI). Chromium poisoning in humans is mainly caused by ingestion of Cr (VI) in the gastrointestinal tract and lungs [66] and, to a lesser extent, through intact skin [67]. Notably, several biotoxic effects, including on the liver, kidneys, and hematologic system, may be caused by high exposure to Cr (VI) [64, 68]. Prolonged exposure to high levels of chromium has also been associated with an increased risk of certain cancers [25, 45]. To ensure overall well-being, it is crucial to maintain a balanced intake of chromium from seaweed and other dietary sources, avoiding excessive consumption to prevent potential toxicity and adverse health outcomes. The higher mean Cr concentration was found in G. lemaneiformis (0.59 ± 0.30 mg/kg) than in H. musciformes (0.40 ± 0.22 mg/kg). The present study shows a lower concentration of Cr than Siddique et al. [8], Ali et al. [22], and Malea and Kevrekidis [48] (Table 2).
3.3 Estimated daily intake (EDI)
The calculation of the EDI was conducted to assess both the significant non-carcinogenic risk effect and the carcinogenic health impact associated with the consumption of contaminated aquatic foods, as outlined by Liu et al. [69]. The EDI calculation follows the guidelines of the oral reference dose (Rfd) specific to each metal content, which determines the threshold for toxic metal element response and ensures the protection of public health, according to Baki et al. [70]. Table 3 presents the EDIs for adults and children, corresponding to the consumption of the investigated species. The observed EDIs followed the descending order: Fe > Ca > Zn > Cu > Mn > Ni > Pb > Cr and were compared against the Recommended Daily Allowance (RDA) proposed by WHO [71]. When the EDIs were lesser than the respective RDA for specific metal elements, it indicated a negligible threat to human health through ingestion, as indicated by Ahmed et al. [31]. However, it is important to note that considering it solely as an "acceptable range" or "unacceptable range" based on being lower than the RDA/RfD would not be wise, as cautioned by Baki et al. [70].
3.4 Non-carcinogenic risk assessment
The results of the calculated THQ for the consumption of the investigated fish species have been depicted in Table 3. The THQ values for both adults and children were observed in the following descending order: Fe > Pb > Ni > Cu > Zn > Mn > Ca > Cr. In adults, the range of THQ values ranged from 1.57E−06 to 8.49E−03, while in children, it ranged from 3.50E−05 to 3.78E−02. On average, children had nearly four times higher THQ values compared to adults. However, all THQ values were lower than the threshold limit of 1. THQ values below 1 indicate that the exposure levels are also below the reference limit. Therefore, the lifetime consumption of such fish species would not harm human health, as stated by Yi et al. [72]. Nevertheless, assessing the HI is essential as it indicates the alarming condition of the non-carcinogenic effects on public health, as suggested by Liu et al. [69].
The estimated HI, representing the total THQ, was 1.93E−02 for adults and 8.61E−02 for children. The HI values were also below 1, indicating that the local consumers were protected from non-carcinogenic impacts. These findings align with a study by Khalil et al. [73] where THQ and HI values were within safe conditions, below 1. However, it is essential to note that while THQ and HI are useful tools for risk assessment, they do not directly measure the actual combined impacts of multiple contaminants on human health, as cautioned by Li et al. [74]. Our result was in line with the findings from Chen et al. where local people are risk-free for some metal contents (Al, Mn, As, Ni, Cr, Cu, Hg, Cd, Pb, and Se) due to seaweed consumption. Similar findings were reported by Roleda et al. [75].
3.5 Carcinogenic risk (CR) assessment
Carcinogenic Risk (CR) was evaluated specifically for Pb only, as the oral carcinogenic slope factor is available for Pb among the other investigated substances. The range of CR values in adults ranged from 2.38E−08 to 2.61E−08, while in children, it ranged from 1.06E−07 to 1.16E−07, as shown in Table 3. Among all the species, Gracilaria lemaneiformis exhibited the highest CR value in the children group, indicating that children are relatively more susceptible to carcinogenic risk effects, particularly from the consumption of this species. However, the cumulative CR values did not exceed the threshold range of 10–6 to 10–4. Therefore, our CR findings do not generate any significant carcinogenic risk to the local consumers. These findings are consistent with the findings of Arisekar et al. [76] and Ali et al. [22], who stated that their CR values were within an acceptable range near the Thamirabarani River and Sudanese Red Sea Coast, respectively. In another study, Peng et al. [23] reported that As and Cr in carcinogenic risk assessment exceeded the acceptable levels. These elements were identified as the limiting factors, meaning they could pose the most significant risk regarding potential carcinogenic effects associated with consuming seaweeds. It is important to note that spontaneous climate change, rapid industrialization, and economic activities can increase the amount of metal content in the study area, posing a potential threat to carcinogenic risk issues.
The results of this study have significant ramifications for Bangladesh's and other countries' control of seaweed consumption and cultivation. The presence of HMs in seaweed samples highlights the need for regular monitoring and assessment of seaweed farming sites to ensure that they are not contaminated with harmful substances. During farming, measures should be implemented to prevent the accumulation of HMs in seaweed tissues. This may involve monitoring water quality, site selection, and implementing sustainable farming practices to reduce the risk of HM contamination. Additionally, there is a need for further research on the sources of HM contamination in seaweed culture sites to mitigate the risks associated with HM exposure effectively. Regulatory agencies should implement measures to ensure that seaweed products meet safety standards and are free from harmful contaminants. Studies focusing on the bioaccumulation and transfer of HMs from seaweeds to higher trophic levels could provide valuable insights into the environmental implications of HM contamination in seaweed farms. Additionally, research on the potential health effects of consuming seaweed products with varying levels of HM contamination could help to develop guidelines for safe consumption. Stakeholders may contribute to the seaweed industry's sustainable growth while preserving consumer and environmental safety by addressing these issues.
4 Conclusion
Seaweeds have a long history of being used for a wide range of purposes, including the manufacture of agar, alginates, carrageenan, and furcellaran as well as soil manure and energy production in agriculture. Furthermore, they serve as key ingredients in beauty products and medicines. Seaweeds have a long-standing tradition of being harnessed for their multitude of benefits. With their high demand on the global market, many countries are now actively engaged in seaweed cultivation. In Bangladesh, extensive research is ongoing to establish successful seaweed cultivation practices.
The specific focus of this research was the cultivation of H. musciformes and G. lemaneiformis in the Rezu Khal region. The aim was to evaluate their growth rates and the potential for cultivation in this area. Analysis revealed the presence of various minerals such as Ca, Mg, and Fe within these seaweed species. Notably, certain HMs were also detected in the cultivated seaweed samples. The estimated THQ and CR revealed that the study area was free from non-carcinogenic and carcinogenic risks due to the consumption of aquatic food items.
The findings underscore the importance of understanding the impact of pollution and the seaweed's capacity to absorb HMs, both in natural and cultivated settings. This understanding is essential to guarantee the goods' quality and safety as well as to handle any possible environmental effects, particularly as seaweed farming grows.
Data availability
All data supporting the findings of this study are available within the paper.
References
Bhuyan MS, Haider SMB, Kunda M, Husain SA, Chowdhury E, Senapathi V, Sivakumar K, Elangovan M. Experimental cultivation of seaweed on the Coast of Cox’s Bazar, Bangladesh: identifying the effects of environmental parameters on seaweed growth. J Appl Life Sci Environ. 2023;56:413–36.
Bhuyan MS, Haider SMB, Kundu M, Husain SKA, Chowdhury E. Challenges in cultivation of seaweed: a recent experience from RezuKhal, Cox’s Bazar Coast, Bangladesh. J Oceanogr Marine Environ Syst. 2023;7:01–9.
Bhuyan MS, Haider SMB, Kunda M, Islam MT, Husain SA, Chowdhury E, Mojumder IA (2023). Is seaweed culture a sustainable approach for climate change adaptation? J NOAMI, In press.
Cui Y, Liu X, Li S, Hao L, Du J, Gao D, Kang Q, Lu J. Extraction, characterization and biological activity of sulfated polysaccharides from seaweed Dictyopteris divaricata. Int J Biol Macromol. 2018;117:256–63. https://doi.org/10.1016/j.ijbiomac.2018.05.134.
Bhuyan MS. Ecological risks associated with seaweed cultivation and identifying risk minimization approaches. Algal Res. 2023;69: 102967.
Bhuyan MS, Abid Husain S, Chowdhury E, Bat L. Assessment of carbon sequestration capacity of seaweed in climate change mitigation. J Clim Change. 2022;8(1):1–8.
Bhuyan M, Islam M, Sharif ASM, Hoq M. Seaweed: a powerful tool for climate change mitigation that provides various ecological services. Bangladesh II: Climate Change Impacts Mitig Adapt Dev Ctries. 2021:159–192.
Siddique MAM, Hossain MS, Islam MM, Rahman M, Kibria G. Heavy metals and metalloids in edible seaweeds of Saint Martin’s Island, Bay of Bengal, and their potential health risks. Mar Pollut Bull. 2022;181: 113866.
Arulkumar A, Nigariga P, Paramasivam S, Rajaram R. Metals accumulation in edible marine algae collected from Thondi coast of Palk Bay, Southeastern India. Chemosphere. 2019;221:856–62.
Murai U, Yamagishi K, Kishida R, Iso H. Impact of seaweed intake on health. Eur J Clin Nutr. 2021;75(6):877–89.
FAO, 2018. The State of World Fisheries and Aquaculture 2018 - Meeting the Sustainable Development Goals. Licence: CC BY-NC-SA 3.0 IGO, Rome.
Chowdhury MSN, Hossain MS, AftabUddin S, Alamgir M, Sharifuzzaman SM. Seaweed aquaculture in Bangladesh: present status, challenges and future prospects. Ocean Coast Manag. 2022;228: 106309.
Farhaduzzaman AM, Khan MS, Hasan M, Islam R, Osman MH, Shovon MNH, Haider SM, Kunda M, Islam MT, Bhuyan MS. Seaweed culture, post-harvest processing, and market generation for employment of coastal poor communities in Cox’s Bazar. J Appl Life Sci Environ. 2023;56(2):231–44.
Bhuyan MS, Sharif ASM, Islam MM, Mojumder IA, Das M, Islam MS. Blue economy and the prospect of seaweed in Bangladesh. J Marine Sci Res Oceanogr. 2020;3:1–2.
Hossain MS, Sharifuzzaman SM, Nobi MN, Chowdhury MSN, Sarker S, Alamgir M, Uddin SA, Chowdhury SR, Rahman MM, Rahman MS, Sobhan F. Seaweeds farming for sustainable development goals and blue economy in Bangladesh. Mar Policy. 2021;128: 104469.
Hossain MB, Shanta TB, Ahmed AS, Hossain MK, Semme SA. Baseline study of heavy metal contamination in the Sangu River estuary, Chattogram, Bangladesh. Mar Pollut Bull. 2019;140:255–61.
Hossain MB, Semme SA, Ahmed ASS, Hossain MK, Porag GS, Parvin A, Shanta TB, Senapathi V, Sekar S. Contamination levels and ecological risk of heavy metals in sediments from the tidal river Halda, Bangladesh. Arab J Geosci. 2021;14:1–12.
Pandit D, Haque MM, Saifullah MK, Bhuyan MS, Ali MM, Harun-Al-Rashid A, Uddin MS, Kunda M. Distribution, source identification, and contamination level of trace metals in the sediment of the Shari-Goyain River in Bangladesh: Implications for ecological health risks. J Hazard Mater Adv. 2024;14: 100434.
Filippini M, Baldisserotto A, Menotta S, Fedrizzi G, Rubini S, Gigliotti D, Valpiani G, Buzzi R, Manfredini S, Vertuani S. Heavy metals and potential risks in edible seaweed on the market in Italy. Chemosphere. 2021;263: 127983.
Bhuyan MS, Haider SMB, Meraj G, Bakar MA, Islam MT, Kunda M, Siddique MAB, Ali MM, Mustary S, Mojumder IA, Bhat MA. Assessment of heavy metal contamination in beach sediments of Eastern St. Martin’s Island, Bangladesh: implications for environmental and human health risks. Water. 2023;15(13):2494.
Shah, S. B. (2021). Heavy metals in the marine environment—an overview. Heavy Metals in Scleractinian Corals, 1–26.
Ali AY, Idris AM, Eltayeb MA, El-Zahhar AA, Ashraf IM. Bioaccumulation and health risk assessment of toxic metals in red algae in Sudanese Red Sea coast. Toxin Rev. 2021;40(4):1327–37.
Peng Z, Guo Z, Wang Z, Zhang R, Wu Q, Gao H, Wang Y, Shen Z, Lek S, Xiao J. Species-specific bioaccumulation and health risk assessment of heavy metal in seaweeds in tropic coasts of South China Sea. Sci Total Environ. 2022;832: 155031.
Rahman MS, Hossain MB, Babu SOF, Rahman M, Ahmed AS, Jolly YN, Choudhury TR, Begum BA, Kabir J, Akter S. Source of metal contamination in sediment, their ecological risk, and phytoremediation ability of the studied mangrove plants in ship breaking area, Bangladesh. Marine Pollut Bull. 2019;141:137–46.
Rahman MS, Ahmed ASS, Rahman MM, Babu SOF, Sultana S, Sarker SI, Awual R, Rahman MM, Rahman M. Temporal assessment of heavy metal concentration and surface water quality representing the public health evaluation from the Meghna River estuary, Bangladesh. Appl Water Sci. 2021;11(7):121.
Babu SOF, Hossain MB, Rahman MS, Rahman M, Ahmed AS, Hasan MM, Rakib A, Emran TB, Xiao J, Simal-Gandara J. Phytoremediation of toxic metals: a sustainable green solution for clean environment. Appl Sci. 2021;11(21):10348.
Bánfalvi G. Heavy metals, trace elements and their cellular effects. Cellular effects of heavy metals. 2011, 3–28.
Balali-Mood M, Naseri K, Tahergorabi Z, Khazdair MR, Sadeghi M. Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front Pharmacol. 2021. https://doi.org/10.3389/fphar.2021.643972.
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol. 2014;7(2):60.
Roy JS, Chatterjee D, Das N, Giri AK. Substantial evidences indicate that inorganic arsenic is a genotoxic carcinogen: a review. Toxicol Res. 2018;34:311–24.
Ahmed AS, Rahman M, Sultana S, Babu SOF, Sarker MSI. Bioaccumulation and heavy metal concentration in tissues of some commercial fishes from the Meghna River Estuary in Bangladesh and human health implications. Mar Pollut Bull. 2019;145:436–47.
Ahmed AS, Sultana S, Habib A, Ullah H, Musa N, Hossain MB, Rahman MM, Sarker MSI. Bioaccumulation of heavy metals in some commercially important fishes from a tropical river estuary suggests higher potential health risk in children than adults. PLoS ONE. 2019;14(10): e0219336.
Kubra K, Mondol AH, Ali MM, Islam MS, Akhtar S, Ahmed AS, Bhuyan MS, Rahman MM, Siddique MA, Islam ARMT. Assessment of As, Cr, Cd, and Pb in urban surface water from a subtropical river: contamination, sources, and human health risk. Int J Environ Anal Chem. 2023;1–21.
Bruno Lemos B, Letícia Ramos N, Fernando B. Determination of essential (Ca, Fe, I, K, Mo) and toxic elements (Hg, Pb) in Brazilian rice grains and estimation of reference daily intake. Food Nutr Sci. 2012;2012.
USEPA (2000) Guidance for assessing chemical contaminant data for use in fish advisories. Volume 2: Risk assessment and fish consumption limits, 3rd ed. http://water.epa.gov/scitech/swguidance/fishshellfish/techguidance/risk/upload/2009_04_23_fish_advice_volume2_v2cover.pdf
Griboff J, Wunderlin DA, Monferran MV. Metals, As and Se determination by inductively coupled plasma-mass spectrometry (ICP-MS) in edible fish collected from three eutrophic reservoirs. Their consumption represents a risk for human health? Microchem J. 2017;130:236–44.
USEPA, Risk-Based Concentration Table. (U.S. Environmental Protection Agency, Washington, DC, 2008).
USEPA, 2022. Exposure Factors Handbook 2011 Edition (Final). http://cfpub.epa.gov/. Accessed 31 Dec 2022.
Heshmati A, Sadati R, Ghavami M, Mousavi Khaneghah A. The concentration of potentially toxic elements (PTEs) in muscle tissue of farmed Iranian rainbow trout (Oncorhynchus mykiss), feed, and water samples collected from the west of Iran: a risk assessment study. Environ Sci Pollut Res. 2019;26:34584–93.
Ahmed MF, Mokhtar MB, Alam L, Mohamed CAR, Ta GC. Non-carcinogenic health risk assessment of aluminium ingestion via drinking water in Malaysia. Expo Health. 2019;11:167–80.
Abtahi M, Fakhri Y, Oliveri Conti G, Ferrante M, Taghavi M, Tavakoli J, Heshmati A, Keramati H, Moradi B, Amanidaz N, Mousavi Khaneghah A. The concentration of BTEX in the air of Tehran: a systematic review-meta analysis and risk assessment. Int J Environ Res Public Health. 2018;15(9):1837.
Saha N, Rahman MS, Ahmed MB, Zhou JL, Ngo HH, Guo W. Industrial metal pollution in water and probabilistic assessment of human health risk. J Environ Manage. 2017;185:70–8.
Zhou Y, Jiang D, Ding D, Wu Y, Wei J, Kong L, Long T, Fan T, Deng S. Ecological-health risks assessment and source apportionment of heavy metals in agricultural soils around a super-sized lead-zinc smelter with a long production history, in China. Environ Pollut. 2022;307: 119487.
Hossain MB, Ahmed ASS, Sarker MSI. Human health risks of Hg, As, Mn, and Cr through consumption of fish, Ticto barb (Puntius ticto) from a tropical river, Bangladesh. Environ Sci Pollut Res. 2018;25(31):31727–36.
Ahmed ASS, Hossain MB, Babu SMOF, Rahman M, Sun J, Sarker MSI. Spatial distribution, source apportionment, and associated risks of trace metals (As, Pb, Cr, Cd, and Hg) from a subtropical river, Gomti, Bangladesh. Int J Sediment Res. 2022;37(1):83–96.
Bremner I, Beattie JH. Metallothionein and the trace minerals. Annu Rev Nutr. 1990;10(1):63–83.
Smith CS, Branham CW, Marquardt BJ, Mann KR. Oxygen gas sensing by luminescence quenching in crystals of Cu (xantphos)(phen)+ complexes. J Am Chem Soc. 2010;132(40):14079–85.
Malea P, Kevrekidis T. Trace element patterns in marine macroalgae. Sci Total Environ. 2014;494:144–57.
WHO. Permissible limits of heavy metals in soil and plants. Geneva: World Health Organization; 1996.
Al-Fartusie FS, Mohssan SN. Essential trace elements and their vital roles in human body. Indian J Adv Chem Sci. 2017;5(3):127–36.
Yoganandham ST, Raguraman V, Muniswamy G, Sathyamoorthy G, Renuka RR, Chidambaram J, Rajendran T, Chandrasekaran K, Ravindranath RRS. Mineral and trace metal concentrations in seaweeds by microwave-assisted digestion method followed by quadrupole inductively coupled plasma mass spectrometry. Biol Trace Element Res. 2019;187(2):579–85.
Goldhaber SB. Trace element risk assessment: essentiality vs. toxicity. Regulat Toxicol Pharmacol. 2003;38(2):232–42.
Mehri A. Trace elements in human nutrition (II)–an update. Int J Prev Med. 2020;11:2.
Shukla AK, Behera SK, Pakhre A, Chaudhari SK. Micronutrients in soils, plants, animals and humans. Indian J Fertilisers. 2018;14(3):30–54.
Anbazhagan V, Partheeban EC, Arumugam G, Arumugam A, Rajendran R, Paray BA, Al-Sadoon MK, Al-Mfarij AR. Health risk assessment and bioaccumulation of metals in brown and red seaweeds collected from a tropical marine biosphere reserve. Marine Pollut Bull. 2021;164: 112029.
Abdallah MA. Heavy metal monitoring in marine seaweeds from the southeastern Mediterranean Sea off the Egyptian coast, 2006–2009. In: Symposium on Marine Vegetation. 2010, 11
Cempel M, Nikel GJPJS. Nickel: A review of its sources and environmental toxicology. Pol J Environ Stud. 2006;15(3):375–82.
Ahmed ASS, Hossain MB, Semme SA, Babu SMOF, Hossain K, Moniruzzaman M. Accumulation of trace elements in selected fish and shellfish species from the largest natural carp fish breeding basin in Asia: a probabilistic human health risk implication. Environ Sci Pollut Res. 2020;27:37852–65.
Ahmed AS, Hossain MB, Babu SOF, Rahman MM, Sarker MSI. Human health risk assessment of heavy metals in water from the subtropical river, Gomti, Bangladesh. Environ Nanotechnol Monit Manag. 2021;15: 100416.
Flora G, Gupta D, Tiwari A. Toxicity of lead: a review with recent updates. Interdiscip Toxicol. 2012;5(2):47–58.
FAO and WHO. 2022. Report of the expert meeting on food safety for seaweed – Current status and future perspectives. Rome, 28–29 0ctober 2021. Food Safety and Quality Series No. 13. Rome. https://doi.org/10.4060/cc0846en
CEVA.2014. Edible seaweed and French regulation- Synthesis made by CEVA (31/03/2014). Cork, Ireland. cybercolloids.net/sites/default/files/seaweedandregulation2014.pdf
Kolo MT, Khandaker MU, Amin YM, Abdullah WHB, Bradley DA, Alzimami KS. Assessment of health risk due to the exposure of heavy metals in soil around mega coal-fired cement factory in Nigeria. Re Phys. 2018;11:755–62.
Khandaker MU, Nasir NLM, Zakirin NS, Kassim HA, Asaduzzaman K, Bradley DA, Zulkifli MY, Hayyan A. Radiation dose to the Malaysian populace via the consumption of bottled mineral water. Radiat Phys Chem. 2017;140:173–9.
Food Safety Authority of Ireland (FSAI).2020. Safety considerations of seaweed and seaweed-derived foods available on the Irish Market. Report of the Scientific Committee of the Food Safety Authority of Ireland (FSAI). Dublin. fsai.ie/SafetyConsiderations_SeaweedAndSeaweedDerivedFoods_IrishMarket
US EPA. Department of Health & Human Services-Agency for Toxic Substances and Disease Registry (ATSDR). In Toxicol. Profile Asbestos; 2001. https://www.atsdr.cdc.gov/toxprofiles/tp61.pdf. Accessed 9 Feb 2021.
Dwyer JT, Allison DB, Coates PM. Dietary supplements in weight reduction. J Am Diet Assoc. 2005;105(5):80–6.
Barua T, SaifulIslamBhuian AKM, Hossain S, Deb N, Ahmed M, Rashid M, Khandaker MU. The presence of radioactive and metal contaminants in wild mushrooms grown in Chattogram hill tracts, Bangladesh. J Radioanal Nuclear Chem. 2019;322(1):173–82.
Liu J, Li Y, Li D, Wang Y, Wei S. The burden of coronary heart disease and stroke attributable to dietary cadmium exposure in Chinese adults, 2017. Sci Total Environ. 2022;825: 153997.
Baki MA, Hossain MM, Akter J, Quraishi SB, Shojib MFH, Ullah AA, Khan MF. Concentration of heavy metals in seafood (fishes, shrimp, lobster and crabs) and human health assessment in Saint Martin Island, Bangladesh. Ecotoxicol Environ Saf. 2018;159:153–63.
USEPA, 2002. Supplemental Guidance for Developing Soil Screening Levels for Superfund Sites OSWER 9355.4–24; USEPA: Washington, DC, USA, 2002.
Yi Y, Yang Z, Zhang S. Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environ Pollut. 2011;159(10):2575–85.
Khalil A, Jamil A, Khan T. Assessment of heavy metal contamination and human health risk with oxidative stress in fish (Cyprinus carpio) from Shahpur Dam, Fateh Jang, Pakistan. Arab J Geosci. 2020;13:1–10.
Li R, Kuo YM, Liu WW, Jang CS, Zhao E, Yao L. Potential health risk assessment through ingestion and dermal contact arsenic-contaminated groundwater in Jianghan Plain, China. Environ Geochem Health. 2018;40:1585–99.
Roleda MY, Marfaing H, Desnica N, Jónsdóttir R, Skjermo J, Rebours C, Nitschke U. Variations in polyphenol and heavy metal contents of wild-harvested and cultivated seaweed bulk biomass: health risk assessment and implication for food applications. Food Control. 2019;95:121–34.
Arisekar U, Shakila RJ, Shalini R, Jeyasekaran G. Human health risk assessment of heavy metals in aquatic sediments and freshwater fish caught from Thamirabarani River, the Western Ghats of South Tamil Nadu. Mar Pollut Bull. 2020;159: 111496.
Acknowledgements
Special thanks to the Business Promotion Council (BPC), Ministry of Commerce for financial support. The authors express heartfelt thanks to Bangladesh Marine Fisheries Association (BMFA) for this research facility. Thanks to the Biological Oceanography Lab and the Bangladesh Oceanographic Research Institute for their technical support. The authors are grateful to the Bangladesh Council of Scientific and Industrial Research (BCSIR), Chittagong, for providing the necessary analysis facilities.
Funding
We receive funding from Business Promotion Council (BPC), Ministry of Commerce and partial funding from Bangladesh Marine Fisheries Association (BMFA).
Author information
Authors and Affiliations
Contributions
Md. S. Bhuyan: Conceptualization, supervision, culture, analysis, writing—original draft, review and editing, visualization; M. Kunda: Supervision, review and editing; M.A. Bakar: Analysis; S.K.A. Husain: Review and editing; E. Chowdhury: Review and editing; M.M. Ali: Analysis, review and editing; D. Pandit: Writing—original draft, review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
We do not need any ethical permission for this study. All authors approved the final manuscript and agreed to participate.
Consent for publications
All authors approved the final manuscript and the submission to this journal.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bhuyan, M.S., Kunda, M., Bakar, M.A. et al. Heavy metal and mineral analysis of cultivated seaweeds from Cox’s Bazar Coast, Bay of Bengal, Bangladesh: a human health risk implication. Discov Oceans 1, 11 (2024). https://doi.org/10.1007/s44289-024-00012-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44289-024-00012-x