Abstract
Traditionally, the root system has been regarded as the primary component influencing citrus tolerance. Aerial tissues also play a crucial role in abiotic stress tolerance, as they are responsible for vital physiological processes, such as photosynthesis and transpiration. In addition, these tissues are directly exposed to various stress conditions, including extreme temperatures (heat and cold), high light irradiation, and ultraviolet (UV) exposure. In the current climate change scenario, optimizing both citrus rootstocks and grafted scions is crucial to ensure fruit quality and crop yield. Various approaches have been used to investigate the significance of aerial tissues, including in vitro systems, isolated aerial tissue growth, reciprocal grafting, and girdling. This review highlights recent research on the role of aerial tissues in citrus plants under various abiotic stress conditions. Studying and optimizing the genotypes used as scions in grafted citrus plants under abiotic stress conditions is crucial and may contribute to the development of new crop management strategies and breeding programs. Furthermore, this knowledge could be extended to other crops, enabling the development of more resilient and productive agricultural systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Both biotic and abiotic stress conditions significantly affect citrus productivity. Environmental conditions, such as drought, high temperatures, soil salinity, and nutrient deficiencies, negatively affect plant growth, yield, and crop quality. Understanding the effects of stress on citrus plants is crucial for minimizing their adverse effects, fostering sustainable agricultural practices that promote the adaptability and resilience of crops, and improving productivity, particularly in the face of climate change and resource scarcity. The citrus industry is one of the most economically important fruit production sectors in the Mediterranean and subtropical regions. In 2021, citrus fruits ranked as the second most produced fruit worldwide, with a total production of 161.8 million tons, cultivated over 10.2 million hectares (Food and Agriculture Organization [FAO] of the United Nations 2023; available online: http://www.fao.org/faostat/en/#data). Commercial citrus varieties are cultivated by grafting onto specific rootstocks selected according to the geographic region or environmental conditions. Grafting involves joining the root system (rootstock) of one genotype with the shoot system (scion) of another genotype. Tissue regeneration allows the fusion of these two parts, resulting in a single plant sharing a unified vascular system. Grafting has been widely used to improve plant tolerance to biotic and abiotic stressors (Brumós et al. 2010; Colla et al. 2010; Baron et al. 2018; Baron et al. 2019). Grafted plants demonstrate improved resilience to various adverse climatic conditions, including drought, heat, salinity, and diseases. Grafting onto resilient rootstocks enhances water-use efficiency, nutrient uptake, and pathogen resistance in plants, enabling them to withstand changing climatic conditions (Sánchez-Rodríguez et al. 2016; Rouphael et al. 2018). Recent studies have demonstrated that rootstock genotypes influence shoot metabolic composition, leaf shape, and vigor (Gautier et al. 2018; Migicovsky et al. 2019). In addition, the metabolome of leaves in grafted scions is influenced by these factors (Tedesco et al. 2021; Loupit et al. 2022). Harris et al. (2023) reported a significant influence of rootstock genotype on the scion transcriptome and vice versa.
Plants, as sessile organisms, have evolved a range of sensors and receptors that enable them to perceive and respond to subtle alterations in the surrounding environment (Novaković et al. 2018; Amir et al. 2019; Mukarram et al. 2021). Perceived stress signals directly modify the structure or activity of sensors, initiating signal transduction. These sensors play crucial roles in monitoring and regulating the physiological and morphological responses of plants to adverse conditions (Vu et al. 2019; Lamers et al. 2020). Environmental stress affects different plant organs and tissues, leading to various responses at the molecular, cellular, and morphological levels. These responses can vary depending on the tissue and stage of plant development. The root is typically the first organ to experience salt stress, whereas leaves are the first to detect high light intensity, ultraviolet-B (UV-B) and UV-C radiation, extreme ambient temperatures, water deficit or drought conditions, and air pollutants (Podar and Maathuis 2022).
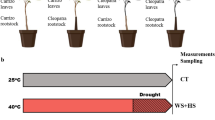
Several approaches are available for investigating the significance of aerial tissues in plant responses to stress (Fig. 1). Studying grafted plants under stressful conditions offers valuable insights into the response and resilience of citrus plants to environmental challenges. The specific effects of scions on a grafted plant can be determined by subjecting both grafted and non-grafted plants to the same stress conditions and analyzing their physiological and molecular responses (Vives-Peris et al. 2023). A metabolic study of grafted citrus revealed alterations in both the sap composition of the plant parts and fruit juice. These changes were attributed to the influence of the rootstock and the interaction between the rootstock and scion (Tietel et al. 2020). Differences in tolerance or sensitivity to biotic or abiotic stress among genotypes are not limited to their aerial parts or root systems. However, determining the specific contribution of each organ to plant tolerance or sensitivity to a particular stress factor under real-world conditions is challenging. Small RNA molecules, including microRNAs and small interfering RNAs, can be transferred between rootstocks and scions in grafted plants. Small RNA molecules can regulate gene expression and potentially influence the physiological processes in recipient tissues (Nakamura et al. 2016). Other studies have demonstrated the movement of proteins between rootstocks and scions. These proteins may have signaling functions and contribute to the coordination of physiological processes in grafted plants (Turnbull and Lopez-Cobollo 2013; Yu et al. 2023). In this context, in vitro tissue culture techniques can address some of these limitations and facilitate the study of genotypic performance. Although artificial from a physiological perspective, in vitro systems enable the culture and maintenance of isolated organs. Several studies have used this approach in plant biology, biochemistry, and molecular biology to investigate the biochemical processes associated with the responses of different genotypes to adverse culture conditions (Montoliu et al. 2009; Ahmad et al. 2020; Dogan 2020; Aazami et al. 2021). Plants constantly adapt their metabolism and development in response to environmental changes through efficient organ-to-organ communication. Long-distance communication between roots and aboveground organs in plants occurs through the xylem (from the root to the aerial part) and phloem (between aboveground organs and from these to the roots), depending on the transport direction of the xylem and phloem. These systems facilitate the transportation of molecules involved in signaling (Kondhare et al. 2021; Sakakibara et al. 2020). Split-root system, grafting, and girdling are suggested methods for studying root-shoot communication and discriminating the nature, direction, and effects of signals between organs in adverse situations. These techniques, when combined with current molecular and genetic tools, can unravel the signaling mechanisms (Asao and Ryan 2015; Castro et al. 2019; Thomas and Frank 2019; Torres et al. 2021).
Schematic representations of various approaches commonly used to investigate the role of aerial tissues in plant stress tolerance: culture of isolated shoots, reciprocal grafting of different genotypes, branch girdling, in vitro tissue culture, bagging of specific organs, and split root systems. Figure was created using Biorender. RH, relative humidity
This study provides an overview of the significance of aerial organs in signaling under abiotic stress conditions and their contribution to plant response and resilience against these constraints, with particular emphasis on citrus plants.
Canopy relevance under water-stress conditions
Drought is a major abiotic stress factor that has detrimental effects on crop productivity and quality. This threat is expected to increase in the near future owing to climate change-induced rainfall reductions and shifts in monsoon patterns (Seleiman et al. 2021). Water stress triggers an array of adverse effects in plants, including the generation of reactive oxygen species (ROS), which results in increased oxidative damage. In response to these challenging conditions, abscisic acid (ABA) signaling orchestrates stomatal closure to prevent tissue dehydration and turgidity loss. However, this advantageous adaptation results in decreased gas-exchange parameters and biomass production. Furthermore, plants defend themselves by synthesizing or accumulating compatible osmolytes, such as the amino acid proline or the conjugate glycine betaine. These osmolytes help regulate the osmotic potential and prevent tissue dehydration (Ahluwalia et al. 2021).
Traditionally, roots have played a significant role in the perception and signaling of water stress. There is a long-standing belief that roots mediate ABA synthesis in response to drought. Synthesized ABA is transported via the xylem to aerial tissues, where it triggers vital responses to specific stimuli. Stomatal closure has been observed in several genotypes (Bharath et al. 2021). However, recent studies have challenged this view. Studies on angiosperm species, including tomatoes, peas, and Arabidopsis, have provided new insights into ABA synthesis and transport mechanisms. Contrary to previous findings, these studies indicate that ABA biosynthesis is primarily localized in the shoots rather than in the roots. This significant discovery highlights the relatively minor contribution of roots to ABA biosynthesis. The enzyme 9-cis-epoxycarotenoid dioxygenase (NCED) plays a central and indispensable role in biosynthesis (McAdam et al. 2016).
Aerial tissues, specifically the leaves, play a significant role in ABA production during water scarcity in citrus plants. This assertion is supported by multiple methodologies. Researchers have conducted short-term water stress experiments lasting 0–5 days to investigate the effects of defoliation on citrus plants under adverse conditions. The results revealed that non-defoliated and partially defoliated plants accumulated ABA in their roots under water-deficit conditions. However, completely defoliated plants exhibited a smaller increase in endogenous ABA levels. This observation highlights the involvement of leaves in ABA biosynthesis and indicates the basipetal transport of ABA in drought-stressed citrus plants (Manzi et al. 2017). Additional investigations have been conducted on citrus plants exposed to simulated water stress using polyethylene glycol (PEG). Plants were maintained at 100% relative humidity by spraying and bagging the canopy. ABA accumulation was exclusively observed in the leaves and roots of plants exposed to ambient relative humidity conditions. No ABA accumulation was observed in plants subjected to PEG-induced water stress, even when the leaves were maintained at full relative humidity. These findings highlight the importance of citrus canopies in the detection of water stress, suggesting that shoot dehydration is necessary for ABA-mediated responses in citrus plants. Furthermore, these results suggest that leaves can influence ABA biosynthesis in roots by regulating the expression of ABA-related genes, such as CsNCED1 or β-carotene hydroxylase1 (CsβCHX1), in the root system (Manzi et al. 2017).
The contribution of aerial tissues as the primary source of ABA during water stress has also been investigated in experiments conducted using girdled and non-girdled Carrizo citrange (Citrus sinensis L. Osbeck × Poncirus trifoliata L. Raf.) rootstocks cultivated in perlite. These plants were subjected to two successive three-day drought periods, with rehydration occurring between each period. The results demonstrated that root ABA levels increased during the initial drought period in girdled plants but not to the extent observed in non-girdled stressed plants. However, ABA accumulation was not observed in the roots during the second water-deprivation period in girdled plants. These findings support the hypothesis that shoot-to-root ABA transport occurs through the phloem (Manzi et al. 2015). Researchers have conducted experiments involving foliar application of deuterated ABA to Carrizo citrange plants under water stress. The results revealed a higher level of deuterated ABA in the roots than in the leaves, providing supplementary evidence for basipetal transport of ABA from the aerial parts of the plant to belowground tissues (Manzi et al. 2015). Despite limited research on this topic, these findings shed light on the intricate mechanisms of ABA signaling and transport in citrus plants, particularly under water stress conditions.
The pivotal role of aerial tissues in citrus plant responses to drought has been confirmed by studies on plant materials cultivated under in vitro conditions. This controlled environment enables the cultivation of isolated rootless shoots, providing a platform for investigating drought responses, specifically in the aerial parts of the plant. Experiments involving the application of PEG to simulate water stress in intact plants and micropropagated shoots (without a root system) yielded comparable patterns of proline and ABA accumulation in both groups. This parallel response reaffirms the prominent contribution of aerial tissues to drought response in citrus plants (Pérez-Clemente et al. 2012). This perspective aligns with findings from field-based investigations of grafted water-stressed plants. These studies focused on Valencia sweet orange (Citrus sinensis L. Osbeck) grafted onto different rootstocks, such as ‘Rangpur Santa Cruz’ lime (Citrus limonia Osbeck) or ‘Sunki Maravilha’ mandarin (Citrus sunki Hayata). The outcomes demonstrated that while ungrafted ‘Rangpur Santa Cruz’ lime was more drought-tolerant than ‘Sunki Maravilha’ mandarin, grafting either rootstock with ‘Valencia’ sweet orange scion resulted in similar physiological, biochemical, and molecular responses under water stress conditions. These findings highlight the significance of aerial tissues in influencing citrus responses to drought (dos Santos et al. 2019).
These results emphasize the significance of aerial tissues in ABA production under drought conditions. This implies a faster response in regulating stomatal closure to mitigate the harmful effects of drought, thereby avoiding the necessity of transporting ABA from the underground tissues. However, further research is required to validate these findings.
Effect of plant aerial tissues on salt stress tolerance
Soil salinity, a major stressor, has worsened in recent decades because of human activities, specifically improper irrigation practices and overexploitation of aquifers. This has resulted in the salinization of arable land areas, which is often driven by seawater intrusion (Hassani et al. 2021). Salt stress negatively affects plant physiology and crop productivity via two primary mechanisms: osmotic imbalance and ionic toxicity. Salt stress disrupts the osmotic balance between plants and soil, impeding water uptake and inducing turgor loss. Salt stress in plants is caused by the accumulation of sodium (Na+) and chloride (Cl−) ions in plant tissues. This accumulation interferes with nutrient uptake, leading to calcium or potassium deficiencies owing to competition with Na+ ions. In addition, essential plant processes are disrupted in the presence of these toxic ions. Among these ions, Cl− is known to contribute significantly to the detrimental effects of salt stress in citrus plants. Its accumulation negatively affects multiple physiological and biochemical processes, including citrus growth, yield, and overall health (López-Climent et al. 2008; Othman et al. 2023).
Citrus plants exhibit tolerance to high soil salinity through a range of physiological and biochemical mechanisms. The accumulation of inorganic and organic solutes in plant cells to counteract the osmotic effects of salt stress has been well-documented (Fernandez-Ballester et al. 1998; López-Climent et al. 2008). This osmotic adjustment maintains cell turgor and prevents water loss, enabling plants to withstand osmotic stress induced by high salt concentrations in the soil. Furthermore, the regulation of ion uptake and transport, specifically Na+ and Cl− ions, is crucial for salt stress tolerance in citrus plants. Thus, tolerant citrus genotypes employ mechanisms that restrict the uptake and translocation of these toxic ions to their aerial tissues, thereby minimizing potential damage (Hussain et al. 2012). Ion regulation is intricately linked to water consumption and transpiration rate. ABA is a key phytohormone. It mediates stomatal closure, reduces transpiration, and concurrently inhibits the uptake and transport of toxic ions, such as Na+ and Cl− (Gómez-Cadenas et al. 2002). Citrus plants employ the strategy of sequestering excess Na+ and Cl− ions into vacuoles with the assistance of transporters located in the tonoplast (Wu and Li 2019).
Previous studies on Carrizo citrange plants subjected to salt stress under in vitro conditions have revealed that, in contrast to water stress conditions, citrus roots are essential for regulating their response to salinity. Plants grown without a root system do not show any accumulation of proline or ABA (Pérez-Clemente et al. 2015). However, studies comparing the development of scions grafted onto different rootstocks have revealed variations in tolerance to salt stress. For instance, ‘Star Ruby’ grapefruit (Citrus paradisi Macfad.) and ‘Tahiti’ acid lime (Citrus latifolia Yu. Tanaka) exhibit different levels of salt stress tolerance depending on the rootstock they are grafted onto (Barbosa Brito et al. 2021). Previous studies have examined the effect of salt stress on more than ten mandarin genotypes cultivated as isolated twigs (50–60 cm) in a solution supplemented with 50 mM NaCl. Water consumption and leaf Cl− content showed a stronger correlation with salt stress sensitivity than other parameters, such as phenotypical symptoms, gas exchange parameters, ion content, and antioxidant status. Among the varieties studied, ‘Willow leaf’ (Citrus deliciosa Ten.), ‘Beauty’ (Citrus tangerina Hort.), and ‘Fuzhu’ (Citrus erythrosa Hort.) exhibited the highest salt tolerance (Ben Yahmed et al. 2016).
These results align with previous experiments conducted on two-year-old grafted citrus plants using combinations of Carrizo citrange or Citrus macrophylla Wester rootstocks, which are known for their sensitivity and tolerance to salt, respectively. The scions used were ‘Navelina’ orange (Citrus sinensis L. Osbeck) or ‘Oronules’ mandarin (Citrus clementina Hort. Ex Tan.). The plants were watered with a solution containing 90 mM NaCl for one month, whereas the control group was not exposed to NaCl. This study revealed that plants grafted with ‘Oronules’ exhibited greater tolerance to salt stress compared to those grafted with ‘Navelina’. This was attributed to reduced stomatal density, which contributed to a lower accumulation of toxic Cl− ions in the leaves without limiting photosynthetic performance (although it was lower under control conditions). Additionally, the study suggested that DTX35.1 and DTX35.2 tonoplast MATE transporters may play a role in the sequestration of Cl− ions, thereby enhancing salt stress tolerance (Vives-Peris et al. 2023). The role of vacuole membrane transporters in citrus plants subjected to high salinity has been investigated in relation to Na+ ions, revealing that the transporters SOS1, NHX1, and HKT1 may be involved in the compartmentalization of this ion. Specifically, NHX1 is primarily involved in shoots, and its expression is enhanced in salt-stressed plants of the Na+ excluder trifoliate orange (Poncirus trifoliata L. Raf. cv. Rubidoux). However, no significant differences in NHX1 expression were observed in the shoots of Na+-sensitive Cleopatra mandarin (Citrus reshni Hort. ex Tan.; Martínez-Alcántara et al. 2015).
Importance of shoots in plants subjected to extreme temperatures
Temperature significantly influences plant development and has a considerable effect on plant physiology and survival, particularly in fruit tree species. Climate change has increased the occurrence and severity of heat and cold waves, leading to significant agricultural losses in terms of crop productivity and fruit quality.
Plant aerial tissues initially sense these temperature changes as the roots are shielded by the soil. Therefore, the photosynthetic apparatus is one of the first systems to respond to adverse conditions. Heat stress typically enhances transpiration and photosynthetic rates in plants, whereas cold stress tends to reduce these vital parameters (Allen and Ort 2001; Repkina et al. 2021; Balfagón et al. 2022a). Furthermore, extreme temperatures, both high and low, can disrupt cell membranes, causing protein denaturation and enzyme deactivation, ultimately compromising plant survival (Wahid and Shabbir 2005). High temperatures increase membrane fluidity and cellular membrane permeability (Dhanda and Munjal 2012). Similarly, cold stress induces alterations in membrane composition, leading to the upregulation of genes associated with fatty acid biosynthesis and phospholipid degradation in grapefruit plants. This adaptive response aims to minimize cellular damage (Maul et al. 2008). At the cellular level, both high- and low-temperature stresses trigger a significant increase in ROS production. Therefore, the response of the antioxidant machinery is crucial for preventing plant damage, especially within chloroplasts. Phytohormones are crucial for improving tolerance to extreme temperatures. For example, ethylene (ET) promotes thermotolerance and primarily reduces ROS in leaves (Poór et al. 2022). Similarly, the addition of exogenous ABA to wheat enhances cold tolerance by increasing the activity of antioxidant enzymes, including catalase (CAT) and superoxide dismutase (SOD) (Yu et al. 2020). Certain strategies are associated with the maintenance of optimal plant hydration because both heat and cold can cause water stress. Therefore, a key adaptation strategy to extreme temperatures is the accumulation of osmoprotective metabolites, including amino acids, organic acids, and sugars.
Citrus is a perennial crop that thrives in tropical and subtropical regions, with optimal growth occurring between 15°C and 32°C. However, extreme temperatures significantly affect citrus plants, leading to a substantial reduction in productivity. Extreme temperatures have the potential to affect various aspects of fruit trees, including their morphology, physiology, and molecular characteristics. In addition, these temperatures can lead to reduced flowering, increased fruit drop, and diminished fruit quality (Li et al. 2023). Recent studies have investigated the physiological and biochemical responses of different citrus genotypes to a combination of water stress and high temperatures. These studies have shown that a temperature of 40ºC induces mild heat stress in citrus crops (Zandalinas et al. 2017; Terán et al. 2023). Citrus plants have developed diverse regulatory pathways and adaptive strategies to thrive in subtropical climates (Tercan and Dereli 2020).
Heat stress
High-temperatures negatively affect the photosynthetic system in citrus, as well as in other species. Understanding the adaptation of these systems is crucial for the development of new plant protection strategies (Allakhverdiev et al. 2008; Hu et al. 2020). High-temperature exposure significantly decreased the net photosynthetic rate and increased ROS levels and antioxidant enzyme activities in Satsuma mandarin (Citrus unshiu Marc.) and Navel orange (Citrus sinensis Osbeck) plants. Guo et al. (2006) proposed two mechanisms for protecting the photosynthetic apparatus: photorespiration and the Halliwell-Asada cycle. High temperatures in Fingered citron (Citrus medica var. sarcodactylis Swingle), a moderately heat-tolerant citrus genotype, disturbs the equilibrium between ROS production and the activity of ROS-scavenging enzymes, resulting in chloroplast damage. This highlights the significance of antioxidant systems (Chen et al. 2012).
Similarly, an increase in ascorbate peroxidase (APX) activity has been observed in two citrus rootstocks under heat stress (Zandalinas et al. 2017). Chlorophyll and carotenoids are crucial metabolites that protect this system. In the Sovage citrange (C. sinensis × Poncirus trifoliata), a significant decrease in the endogenous levels of these metabolites was observed. Conversely, in Brazilian sour oranges (Citrus aurantium), their concentrations remained constant under moderate heat stress, suggesting a higher tolerance (Shafqat et al. 2019). Thus, protection of the photosynthetic apparatus is essential for increasing tolerance to high-temperature stress. Exogenous spermidine application mitigates high temperature-induced damage in citrus plants by enhancing the activity of enzymatic and non-enzymatic antioxidants in leaves (Chao et al. 2022). Another crucial survival strategy under high-temperature conditions is an increase in heat shock protein (HSP) levels. A recent study found that heat stress-tolerant citrus genotypes exhibit higher CsHSP expression, which enables them to sustain high photosynthetic activity by reducing stomatal conductance (Shafqat et al. 2021).
The recent increase in the frequency and severity of heat waves, particularly during the plant reproductive phase, has heightened the need for effective solutions to mitigate the adverse effects of high temperatures and minimize production losses. In this regard, research has focused on evaluating the protective effects of chemical substances, such as kaolin, calcium hydroxide, and calcium silicate (Abd El-Naby et al. 2020a; 2020b; Terán et al. 2023). These substances primarily act by reflecting solar light and reducing leaf temperature during heat stress (Rafie-Rad et al. 2022).
Cold stress
The response to cold stress in citrus fruit has been extensively investigated due to the common practice of using cold storage to extend the postharvest lifespan of fruits (Lafuente and Romero 2022; Kim et al. 2023). However, limited research has been conducted on the responses of aerial plant organs to adverse conditions, particularly in commercial varieties. Researchers have focused exclusively on assessing the tolerance of citrus rootstocks, as they are known to significantly influence responses to biotic and abiotic stressors.
Trifoliate orange (Poncirus trifoliata [L.] Raf) has been extensively studied because of its cold tolerance (Peng et al. 2020). In the study of Wang et al. (2015), 5549 P. trifoliata genes were differentially expressed in response to cold treatment. These genes primarily function in the biosynthesis and regulation of plant hormones (ABA, ET, and gibberellins), polyamine biosynthesis, and ROS production associated with cold-induced signaling (Wang et al. 2015). Prolonged exposure to cold stress in the Carrizo citrange resulted in increased proline levels, which were attributed to the activation of the ornithine aminotransferase pathway (Primo-Capella et al. 2021). Proteomic analysis of Citrus junus, a rootstock widely used in China because of its tolerance to freezing stress, revealed that sugars and secondary metabolism play key roles in cold stress resistance in this plant (Jiang et al. 2021). A comparative transcriptomic analysis of citrus cold-resistant and -sensitive rootstocks showed an upregulation of genes associated with sugar metabolism in the tolerant rootstock, which was accompanied by an increase in free sugars (Primo-Capella et al. 2022). A comparative study between Chongyi wild mandarin (Citrus reticulata), a cold-hardy wild citrus, and Poncirus trifoliata demonstrated that the enhanced cold tolerance of Chongyi could be attributed to increased levels of soluble sugars and greater resistance to high levels of ROS (Peng et al. 2021). He et al. (2020) demonstrated the upregulation of CsPIF8, a phytochrome-interacting transcription factor, under cold stress and investigated its regulatory role in SOD expression.
A recent study evaluated the effect of cold stress on two rootstocks with different tolerance to chilling, as well as their combinations with two citrus varieties, Olinda Valencia orange (Citrus sinensis [L.] Osbeck) and Murcott Tangerino (Citrus reticulata [L.] Blanco) (Hmmam et al. 2023). This study found that Murcott shoots budded onto Volkameriana (Citrus volkameriana) rootstock (cold tolerant) and Macrophylla rootstock (cold sensitive) exhibited higher levels of MDA compared to Olinda. Interestingly, plants grafted with Olinda accumulated a higher proline content than plants grafted with Murcott, regardless of the rootstock used. This suggests the significance of proline accumulation in low-temperature tolerance and highlights the role of aerial plants in this tolerance.
Role of aerial tissues under abiotic stress combinations
In natural environments, plants commonly experience multiple stressors simultaneously rather than isolated stress factors (Soto et al. 2022; Dahro et al. 2023). This highlights the necessity of investigating the effects of specific scenarios on plant responses, because there can be variations across different combinations (Zandalinas et al. 2018).
Limited research has been conducted on the specific role of aerial tissues in the ability of citrus plants to tolerate combinations of abiotic stressors. Although recent studies have explored the effects of multiple stressors on citrus plants, few have focused on the contribution of aerial tissues. Specifically, these studies have focused on the convergence of drought and heat stress, which are two of the most critical challenges in the context of climate change. Balfagón et al. (2022a; 2022b) used reciprocal grafted Carrizo citrange and Cleopatra mandarin plants to investigate the specific roles of different organs under this particular stress combination. These experiments emphasize the importance of rootstocks in conferring tolerance to combined abiotic stresses. Plants grafted with Carrizo citrange exhibited improved resilience to this combination of stressors. Balfagón et al. (2022a) observed reduced foliar damage and elevated photosynthesis rates, resulting in alterations in leaf sugar content. Furthermore, Balfagón et al. (2022b) found that plants grafted with Cleopatra mandarin (a sensitive scion) showed increased hydrogen peroxide levels and reduced activities of key antioxidant enzymes (APX, CAT, and SOD) compared to plants grafted with Carrizo citrange. Recent studies have examined the use of palliative kaolin treatments in Carrizo citrange plants exposed to a combination of heat and high-intensity light. These studies demonstrate that applying kaolin directly to the aerial parts of plants can help maintain the photosynthetic system and levels of chlorophyll and carotenoids. Additionally, kaolin treatment improves the ABA-mediated signaling response in plants under this stress combination, potentially enhancing the observed tolerance (Terán et al. 2023).
These findings confirm the significant role of aerial tissues in conferring tolerance to these specific stress combinations. These tissues are the primary sites for carbon fixation, promoting the synthesis of sugars and enhancing the ability of plants to tolerate these challenging conditions. Further research is required to fully understand the significance of citrus shoots in their tolerance to the combined stress of drought and high temperatures. Although these studies offer some insights, investigation of the mechanisms by which citrus shoots respond to various stress conditions beyond this specific combination is necessary. Additionally, to the best of our knowledge, there is currently a lack of available information on this topic for citrus and other crops.
Concluding remarks
Over the past few decades, the importance of root systems in grafted crops under abiotic stress has been increasingly acknowledged. However, exploring both subterranean organs and the canopy has the potential to increase plant resilience to adverse conditions by improving essential plant processes, such as photosynthesis and transpiration. This review has delved into several illustrative studies that have focused on the role of the canopy in citrus plants cultivated under adverse conditions (Fig. 2). Consequently, optimizing both rootstocks and scions under specific environmental conditions is a promising strategy to boost plant tolerance and increase citrus productivity. This is especially relevant given the current climate change and increasing global population.
The main mechanisms by which the aerial tissues of citrus plants cope with drought, salinity, and extreme temperatures (heat and cold) illustrate their physiological and biochemical responses to these specific stressors. ABA, abscisic acid; HSP, heat shock protein; ROS, reactive oxygen species; CAT, catalase; SOD, superoxide dismutase; APX, ascorbate peroxidase
Availability of data and materials
The data used for the preparation of this article will be made available by the authors under reasonable request.
Change history
12 March 2024
A Correction to this paper has been published: https://doi.org/10.1007/s44281-024-00040-9
References
Aazami MA, Rasouli F, Ebrahimzadeh A. Oxidative damage, antioxidant mechanism and gene expression in tomato responding to salinity stress under in vitro conditions and application of iron and zinc oxide nanoparticles on callus induction and plant regeneration. BMC Plant Biol. 2021;21:597. https://doi.org/10.1186/s12870-021-03379-7.
Abd El-Naby SKM, Abdelkhalek A, Baiea MHM, Amin OA. Impact of spraying some chemical substances on protecting Valencia orange trees from heat stress injuries. Plant Arch. 2020a;20:2265–70.
Abd El-Naby SKM, Abdelkhalek A, Baiea MHM, Amin OA. Mitigation of heat stress effects on Washington navel orange by using melatonin, gibberellin and salicylic acid treatments. Plant Arch. 2020b;20:3523–34.
Ahluwalia O, Singh PC, Bhatia R. A review on drought stress in plants: implications, mitigation and the role of plant growth promoting rhizobacteria. Resour Environ Sustain. 2021. https://doi.org/10.1016/j.resenv.2021.100032.
Ahmad MA, Javed R, Adeel M, Rizwan M, Yang Y. PEG 6000-stimulated drought stress improves the attributes of in vitro growth steviol glycosides production, and antioxidant activities in Stevia rebaudiana Bertoni. Plants. 2020;9:1152. https://doi.org/10.3390/plants9111552.
Allakhverdiev SI, Kreslavski VD, Klimov VV, Los DA, Carpentier R, Mohanty P. Heat stress: an overview of molecular responses in photosynthesis. Photosynth Res. 2008;98:541–50. https://doi.org/10.1007/s11120-008-9331-0.
Allen DJ, Ort DR. Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci. 2001;6:36–42. https://doi.org/10.1016/s1360-1385(00)01808-2.
Amir M, Saeed S, Liu XD, Mannan A, Khan A, Li ZZ, et al. Soil organic content of stands of different ages in a subtropical chir pine (Pinus roxburghii) forest of Pakistan. Appl Ecol Environ Res. 2019;17:11475–87. https://doi.org/10.15666/aeer/1705_114751487.
Asao S, Ryan MG. Carbohydrate regulation of photosynthesis and respiration from branch girdling in four species of wet tropical rain forest trees. Tree Physiol. 2015;35:608–20. https://doi.org/10.1093/treephys/tpv025.
Balfagón D, Rambla JL, Granell A, Arbona V, Gómez-Cadenas A. Grafting improves tolerance to combined drought and heat stresses by modifying metabolism in citrus scion. Environ Exp Bot. 2022a;195:104793. https://doi.org/10.1016/j.envexpbot.2022.104793.
Balfagón D, Terán F, de Oliveira TR, Santa-Catarina C. Citrus rootstocks modify scion antioxidant system under drought and heat stress combination. Plant Cell Rep. 2022b;41:593–602. https://doi.org/10.1007/s00299-021-02744-y.
Barbosa Brito ME, Fernandes PD, Gheyi HR, dos Anjos Soares LA, dos Santos Soares Filho W, Suassuna JF. Screening of citrus scion-rootstock combinations for tolerance to water salinity during seedling formation. Acta Sci Agron. 2021;43:e48163. https://doi.org/10.4025/actasciagron.v43i1.48163.
Baron D, Amaro ACE, Macedo AC, Boaro CSF, Ferreira G. Physiological changes modulated by rootstocks in atemoya (Annona x atemoya Mabb.): gas exchange, growth and ion concentration. Braz J Bot. 2018;41:219–25. https://doi.org/10.1007/s40415-017-0421-0.
Baron D, Amaro ACE, Pina A, Ferreira G. An overview of grafting re-establishment in woody fruit species. Sci Hortic. 2019;243:84–91. https://doi.org/10.1016/j.scienta.2018.08.012.
Ben Yahmed J, de Oliveira TM, Novillo P, Quinones A, Forner MA, Salvador A, et al. A simple, fast and inexpensive method to assess salt stress tolerance of aerial plant part: investigations in the mandarin group. J Plant Physiol. 2016;190:36–43. https://doi.org/10.1016/j.jplph.2015.10.008.
Bharath P, Gahir S, Raghavendra AS. Abscisic acid-induced stomatal closure: an important component of plant defense against abiotic and biotic stress. Fron Plant Sci. 2021;12:615114. https://doi.org/10.3389/fpls.2021.615114.
Brumós J, Talón M, Bouhlal R, Colmenero-Flores JM. Cl- homeostasis in includer and excluder citrus rootstocks: transport mechanisms and identification of candidate genes. Plant Cell Environ. 2010;33:2012–27. https://doi.org/10.1111/j.1365-3040.2010.02202.x.
Castro P, Puertolas J, Dodd IC. Stem girdling uncouples soybean stomatal conductance from leaf water potential by enhancing leaf xylem ABA concentration. Environ Exp Bot. 2019;159:149–56. https://doi.org/10.1016/j.envexpbot.2018.12.020.
Chao X, Tang Y, Liu X, Yang H, Wang Y, Hu Z, et al. Exogenous spermidine enhances the photosynthetic and antioxidant capacity of citrus seedlings under high temperature. Plant Signal Behav. 2022;17:2086372. https://doi.org/10.1080/15592324.2022.2086372.
Chen WR, Zheng JS, Li YQ, Guo WD. Effects of high temperatures on photosynthesis, chlorophyll fluorescence, chloroplast ultrastructure, and antioxidant activities in fingered citron. Rus J Plant Physiol. 2012;59:732–40. https://doi.org/10.1134/S1021443712060040.
Colla G, Rouphael Y, Cardarelli M, Salerno A, Rea E. The efectiveness of grafting to improve the alkalinity tolerance in watermelon. Environ Exp Bot. 2010;68:283–91. https://doi.org/10.1016/j.envexpbot.2009.12.005.
Dahro B, Li C, Liu JH. Overlaping responses to multiple abiotic stresses in citrus: from mechanism understanding to genetic improvement. Hortic Adv. 2023;1:4. https://doi.org/10.1007/s44281-023-00007-2.
Dhanda SS, Munjal R. Heat tolerance in relation to acquired thermotolerance for membrane lipids in bread wheat. Field Crops Res. 2012;135:30–7. https://doi.org/10.1016/j.fcr.2012.06.009.
Dogan M. Effect of salt stress on in vitro organogenesis from nodal explant of Limnophila aromatica (Lamk.) Merr. and Bacopa monnieri (L.) Wettst. and their physio-morphological and biochemical responses. Physiol Mol Biol Plants. 2020;26:803–16. https://doi.org/10.1007/s12298-020-00798-y.
dos Santos IC, de Almeida AAF, Pirovani CP, Costa MGC, da Silva MFGF, Bellete BS, et al. Physiological, biochemical and molecular responses to drought conditions in field-grown grafted and ungrafted citrus plants. Environ Exp Bot. 2019;162:406–20. https://doi.org/10.1016/j.envexpbot.2019.03.018.
Fernandez-Ballester G, Martinez V, Ruiz D, Cerdá A. Changes in inorganic and organic solutes in citrus growing under saline stresses. J Plant Nutr. 1998;21:2497–514. https://doi.org/10.1080/01904169809365582.
Torres LF, López de Andrade SA, Mazzafera P. Split-root, grafting and girdling as experimental tools to study root-to shoot-to root signaling. Environ Exp Bot. 2021;191:104631. https://doi.org/10.1016/j.envexpbot.2021.104631.
Gautier A, Cookson SJ, Hevin C, Vivin P, Lauvergeat V, Mollier A. Phosphorus acquisition efficiency and phosphorus remobilization mediate genotype-specific differences in shoot phosphorus content in grapevine. Tree Physiol. 2018;38:1742–51. https://doi.org/10.1093/treephys/tpy074.
Gómez-Cadenas A, Arbona V, Jacas J, Primo-Millo E, Talon M. Abscisic acid reduces leaf abscission and increases salt tolerance in citrus plants. J Plant Growth Regul. 2002;21:234–40. https://doi.org/10.1007/s00344-002-0013-4.
Guo YP, Zhou HF, Zhang LC. Photosynthetic characteristics and protective mechanisms against photooxidation during high temperature stress in two citrus species. Sci Hortic. 2006;108:260–7. https://doi.org/10.1016/j.scienta.2006.01.029.
Harris ZN, Pratt JE, Kovacs LG, Klein LL, Kwasniewski MT, Londo JP, et al. Grapevine scion gene expression is driven by rootstock and environment interaction. BMC Plant Biol. 2023;23:211. https://doi.org/10.1186/s12870-023-04223-w.
Hassani A, Azapagic A, Shokri N. Global predictions of primary soil salinization under changing climate in the 21st century. Nat Commun. 2021;12:6663. https://doi.org/10.1038/s41467-021-26907-3.
He Z, Zhao T, Yin Z, Liu J, Cheng Y, Xu J. The phytochrome-interacting transcription factor CsPIF8 contributes to cold tolerance in citrus by regulating superoxide dismutase expression. Plant Sci. 2020;298:110584. https://doi.org/10.1016/j.plantsci.2020.110584.
Hmmam I, Abdelaal RA, Gomaa AH. Insight into chilling stress response of key citrus grafting combinations grown in Egypt. Plant Stress. 2023;8:100155. https://doi.org/10.1016/j.stress.2023.100155.
Hu S, Ding Y, Zhu C. Sensitivity and responses of chloroplasts to heat stress in plants. Front Plant Sci. 2020;11:375. https://doi.org/10.3389/fpls.2020.00375.
Hussain S, Luro F, Costantino G, Ollitrault P, Morillon R. Physiological analysis of salt stress behaviour of citrus species and genera: low chloride accumulation as an indicator of salt tolerance. S Afr J Bot. 2012;81:103–12. https://doi.org/10.1016/j.sajb.2012.06.004.
Jiang J, Hou R, Yang N, Li L, Deng J, Qin G, et al. Physiological and TMT-labeled proteomic analyses reveal important roles of sugar and secondary metabolism in Citrus junos under cold stress. J Proteomics. 2021;237:104145. https://doi.org/10.1016/j.jprot.2021.104145.
Kim M, Moon YE, Han SG, Yun SK, Joa JH, Park JS. Impact of cold stress on physiological responses and fruit quality of Shiranuhi mandarin in response Tol cold conditions. Horticulturae. 2023;9:906. https://doi.org/10.3390/horticulturae9080906.
Kondhare KR, Patil NS, Banerjee AK. A historical overview of long-distance signalling in plants. J Exp Bot. 2021;72:4218–36. https://doi.org/10.1093/jxb/erab048.
Lafuente MT, Romero P. Hormone profiling and heat-induced tolerance to cold stress in citrus fruit. Postharvest Biol Technol. 2022;194:112088. https://doi.org/10.1016/j.postharvbio.2022.112088.
Lamers J, van der Meer T, Testerink C. How plants sense and respond to stressful environments. Plant Physiol. 2020;182:1624–35. https://doi.org/10.1104/pp.19.01464.
Li S, Chen H, Yu H, Li Y, Wang L. Responses and adaptations of fruit trees to high temperatures. Fruit Res. 2023;3:23. https://doi.org/10.48130/FruRes-2023-0023.
López-Climent MF, Arbona V, Pérez-Clemente RM, Gómez-Cadenas A. Relationship between salt tolerance and photosynthetic machinery performance in citrus. Environ Exp Bot. 2008;62:176–84. https://doi.org/10.1016/j.envexpbot.2007.08.002.
Loupit G, Fonayet JV, Prigent S, Prodhomme D, Spilmont AS, Hilbert G, et al. Identifying early metabolite markers of successful graft union formation in grapevine. Hortic Res. 2022;9:uhab070. https://doi.org/10.1093/hr/uhab070.
Manzi M, Lado J, Rodrigo MJ, Zacarías L, Arbona V, Gómez-Cadenas A. Root ABA accumulation in long-term water-stressed plants is sustained by hormone transport from aerial organs. Plant Cell Physiol. 2015;56:2457–66. https://doi.org/10.1093/pcp/pcv161.
Manzi M, Pitarch-Bielsa M, Arbona V, Gómez-Cadenas A. Leaf dehydration is needed to induce abscisic acid accumulation in roots of citrus plants. Environ Exp Bot. 2017;139:116–26. https://doi.org/10.1016/j.envexpbot.2017.05.004.
Martínez-Alcántara B, Martínez-Cuenca MR, Quinones A, Iglesias DJ, Primo-Millo E, Forner-Giner MA. Comparative expression of candidate genes involved in sodium transport and compartmentation in citrus. Environ Exp Bot. 2015;111:52–62. https://doi.org/10.1016/j.envexpbot.2014.11.002.
Maul P, McCollum GT, Popp M, Guy CL, Porat R. Transcriptome profiling of grapefruit flavedo following exposure to low temperature and conditioning treatments uncovers principal molecular components involved in chilling tolerance and susceptibility. Plant Cell Environ. 2008;31:752–68. https://doi.org/10.1111/j.1365-3040.2008.01793.x.
McAdam SAM, Sussmilch FC, Brodribb TJ. Stomatal responses to vapour pressure deficit are regulated by high speed gene expression in angiosperms. Plant Cell Environ. 2016;39:485–91. https://doi.org/10.1111/pce.12633.
Migicovsky Z, Harris ZN, Klein LL, Li M, McDermaid A, Chitwood DH, et al. Rootstock effects on scion phenotypes in a ‘chambourcin’ experimental vineyard. Hortic Res. 2019;6:64. https://doi.org/10.1038/s41438-019-0146-2.
Montoliu A, López-Climent MF, Arbona V, Pérez-Clemente RM, Gómez-Cadenas A. A novel in vitro tissue culture approach to study salt stress responses in citrus. Plant Growth Regul. 2009;59:179–87. https://doi.org/10.1007/s10725-009-9401-0.
Mukarram M, Choudhary S, Kurjak D, Petek A, Khan MMA. Drought: sensing, signalling, effects and tolerance in higher plants. Physiol Plant. 2021;172:1291–300. https://doi.org/10.1111/ppl.13423.
Nakamura S, Hondo K, Kawara T, Okazaki Y, Saito K, Kobayashi K, et al. Conferring high-temperature tolerance to nontransgenic tomato scions using graft transmission of RNA silencing of the fatty acid desaturase gene. Plant Biotechnol J. 2016;14:783–90. https://doi.org/10.1111/pbi.12429.
Novaković L, Guo T, Bacic A, Sampathkumar A, Johnson K. Hitting the wall-sensing and signaling pathways involved in plant cell wall remodeling in response to abiotic stress. Plants. 2018;7:89. https://doi.org/10.3390/plants7040089.
Othman YA, Hani MB, Ayad JY, Hilaire RS. Salinity level influenced morpho-physiology and nutrient uptake of young citrus rootstocks. Heliyon. 2023;9:e13336. https://doi.org/10.1016/j.heliyon.2023.e13336.
Peng Z, Bredeson JV, Wu GA, Shu S, Rawat N, Du D, et al. A chromosome-scale reference genome of trifoliate orange (Poncirus trifoliata) provides insights into disease resistance, cold tolerance and genome evolution in Citrus. Plant J. 2020;105:1215–32. https://doi.org/10.1111/tpj.14993.
Peng T, You XS, Guo L, Zhong BL, Mi LF, Chen JM, et al. Transcriptome analysis of Chongyi wild mandarin, a wild species more cold-tolerant than Poncirus trifoliata, reveals key pathways in response to cold. Environ Exp Bot. 2021;184:104371. https://doi.org/10.1016/j.envexpbot.2020.104371.
Pérez-Clemente RM, Montoliu A, Vives-Peris V, Espinoza VM, Zandalinas SI, Gómez-Cadenas A. Roots are necessary for the responses of in-vitro cultured citrus plants to high salinity but not to osmotic stress. Acta Hortic. 2015;1065:1371–8. https://doi.org/10.17660/ActaHortic.2015.1065.173.
Pérez-Clemente RM, Montoliu A, Zandalinas SI, de Ollas C, Gómez-Cadenas A. Carrizo citrange plants do not require the presence of roots to modulate the response to osmotic stress. Sci World J. 2012;795396. https://doi.org/10.1100/2012/795396
Podar D, Maathuis FJM. The role of roots and rhizosphere in providing tolerance to toxic metals and metalloids. Plant Cell Environ. 2022;45:719–36. https://doi.org/10.1111/pce.14188.
Poór P, Nawaz K, Gupta R, Ashfaque F, Khan MIR. Ethylene involvement in the regulation of heat stress tolerance in plants. Plant Cell Rep. 2022;41:675–98. https://doi.org/10.1007/s00299-021-02675-8.
Primo-Capella A, Martínez-Cuenca MR, Gil-Muñoz F, Forner-Giner MA. Physiological characterization and proline route genes quantification under long-term cold stress in Carrizo citrange. Sci Hortic. 2021;276:109744. https://doi.org/10.1016/j.scienta.2020.109744.
Primo-Capella A, Forner-Giner MA, Martínez-Cuenca MR, Terol J. Comparative transcriptomic analyses of citrus cold-resistant vs. sensitive rootstocks might suggest that a relevant role of ABA signaling in triggering cold scion adaption. BMC Plant Biol. 2022;22:209. https://doi.org/10.1186/s12870-022-03578-w.
Rafie-Rad Z, Moradkhani M, Golchin A, Raza T, Eash NS. Abiotic stress management in citrus. In: Gonzatto MP, Santos JS, editors. Citrus research – horticultural and human health aspects. London: IntechOpen; 2022. https://doi.org/10.5772/intechopen.108337.
Repkina N, Ignatenko A, Holoptseva E, Miszalski Z, Kaszycki P, Talanova V. Exogenous methyl jasmonate improves cold tolerance with parallel induction of two cold-regulated (COR) genes expression in Triticum aestivum L. Plants. 2021;10:1421. https://doi.org/10.3390/plants10071421.
Rouphael Y, Kyriacou MC, Colla G. Vegetable grafting: a toolbox for securing yield stability under multiple stress conditions. Front Plant Sci. 2018;8:2255. https://doi.org/10.3389/fpls.2017.02255.
Sakakibara H. Cytokinin biosynthesis and transport for systemic nitrogen signaling. Plant J. 2020;105:421–30. https://doi.org/10.1111/tpj.15011.
Sánchez-Rodríguez E, Romero L, Ruiz JM. Accumulation of free polyamines enhances the antioxidant response in fruits of grafted tomato plants under water stress. J Plant Physiol. 2016;190:72–8. https://doi.org/10.1016/j.jplph.2015.10.010.
Seleiman MF, Al-Suhaibani N, Ali N, Akmal M, Alotaibi M, Refay Y, et al. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants. 2021;10:259. https://doi.org/10.3390/plants10020259.
Shaqfat W, Jaskani MJ, Maqbool R, Khan AS, Ali Z. Evaluation of citrus rootstocks against drought, heat and their combined stress based on growth and photosynthetic pigments. Int J Agric Biol. 2019;22:1001–9. https://doi.org/10.17957/IJAB/15.1160.
Shaqfat W, Jaskani MJ, Maqbool R, Chattha WS, Ali Z, Naqvi SA, et al. Heat shock protein and aquaporin expression enhance water conserving behavior of citrus under water deficits and high temperature conditions. Environ Exp Bot. 2021;104270. https://doi.org/10.1016/j.envexpbot.2020.104270
Soto LS, Segarra-Medina C, Gómez-Cadenas A, López-Climent A, Vives-Peris V, Zandalinas SI. Climate change-associated multifactorial stress combination: a present challenge for our ecosystems. J Plant Physiol. 2022;276:153764. https://doi.org/10.1016/j.jplph.2022.153764.
Tedesco S, Erban A, Gupta S, Kopka J, Fevereiro P, Kragler F, et al. The impact of metabolic scion-rootstock interaction in different grapevine tissues and phloem exudates. Metabolites. 2021;11:349. https://doi.org/10.3390/metabo11060349.
Terán F, Vives-Peris V, López-Climent MF, Gómez-Cadenas A, Pérez-Clemente RM. Palliative effects of kaolin on citrus plants under controlled stress conditions of high temperature and high light intensity. J Plant Growth Regul. 2023; https://doi.org/10.1007/s00344-023-11103-y
Tercan E, Dereli MA. Development of a land suitability model for citrus cultivation using GIS and multicriteria assessment techniques in Antalya province of Turkey. Ecol Indic. 2020;117:106549. https://doi.org/10.1016/j.ecolind.2020.106549.
Thomas HR, Frank MH. Connecting the pieces: uncovering the molecular basis for long-distance communication through plant grafting. New Phytol. 2019;223:582–9. https://doi.org/10.1111/nph.15772.
Tietel Z, Srivastava S, Fait A, Tel-Zur N, Carmi N, Raveh E. Impact of scion/rootstock reciprocal effects on metabolomics of fruit juice and phloem sap in grafted Citrus reticulata. PLoS ONE. 2020;15:e0227192. https://doi.org/10.1371/journal.pone.0227192.
Turnbull CGN, Lopez-Cobollo RM. Heavy traffic in the fast lane: long-distance signalling by macromolecules. New Phytol. 2013;198:33–51. https://doi.org/10.1111/nph.12167.
Vives-Peris V, López-Climent MF, Moliner-Sabater M, Gómez-Cadenas A, Pérez-Clemente RM. Morphological, physiological, and molecular scion traits are determinant for salt-stress tolerance of grafted citrus plants. Front Plant Sci. 2023;14:1145625. https://doi.org/10.3389/fpls.2023.1145625.
Vu KV, Jeong CY, Nguyen TT, Dinh TTH, Lee H, Hong SW. Deficiency of AtGFAT1 activity impairs growth, pollen germination and tolerance to tunicamycin in Arabidopsis. J Exp Bot. 2019;70:1775–87. https://doi.org/10.1093/jxb/erz055.
Wahid A, Shabbir A. Induction of heat stress tolerance in barley seedlings by pre-sowing seed treatment with glycinebetaine. Plant Growth Regul. 2005;46:133–41. https://doi.org/10.1007/s10725-005-8379-5.
Wang M, Zhang X, Liu JH. Deep sequencing-based characterization of transcriptome of trifoliate orange (Poncirus trifoliara (L.) Raf.) in response to cold stress. BMC Genom. 2015;16:555. https://doi.org/10.1186/s12864-015-1629-7.
Wu H, Li Z. The importance of Cl- exclusion and vacuolar Cl- sequestration: Revisiting the role of Cl- transport in plant salt tolerance. Front Plant Sci. 2019;10:1418. https://doi.org/10.3389/fpls.2019.01418.
Yu Y, Wang S, Xu C, Xiang L, Huang W, Zhang X, et al. The β-1,3-glucanase degrades callose at plasmodesmata to facilitate the transport of the ribonucleoprotein complex in Pyrus betulaefolia. Int J Mol Sci. 2023;24:8051. https://doi.org/10.3390/ijms24098051.
Yu J, Cang J, Lu Q, Fan B, Xu Q, Li W, Wang X. ABA enhanced cold tolerance of wheat ‘dn1’ via increasing ROS scavenging system. Plant Signal Behav. 2020;15:e1780403. https://doi.org/10.1080/15592324.2020.1780403.
Zandalinas SI, Balfagón D, Arbona V, Gómez-Cadenas A. Modulation of antioxidant defense system is associated with combined drought and heat stress tolerance in citrus. Front Plant Sci. 2017;8:953. https://doi.org/10.3389/fpls.2017.00953.
Zandalinas SI, Mittler R, Balfagón D, Arbona V, Gómez-Cadenas A. Plant adaptations to the combination of drought and high temperatures. Physiol Plant. 2018;162:2–12. https://doi.org/10.1111/ppl.12540.
Acknowledgements
Not applicable.
Funding
This work was supported by MCIN/AEI/10.13039/501100011033 and the European Union-Next Generation (grant numbers PID2022-137825OB-I00 and TED2021-129795B-I00). Funding was also obtained from Universitat Jaume I (UJI-A2021-10 and UJI-B2022-18).
Author information
Authors and Affiliations
Contributions
VV-P, ML-C, and RP-C collected information and prepared the manuscript. AG-C planned and organized the manuscript. All the authors have contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Authors declare that they do not have commercial or financial interests which could be considered as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Article title is updated, figure 1 and figure 2 has been exchanged.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vives-Peris, V., Pérez-Clemente, R.M., Gómez-Cadenas, A. et al. Involvement of citrus shoots in response and tolerance to abiotic stress. HORTIC. ADV. 2, 3 (2024). https://doi.org/10.1007/s44281-023-00027-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44281-023-00027-y