Abstract
Jasmonic acid (JA) is an important and widely distributed plant hormone. However, the molecular and physiological mechanism of JA in improving drought tolerance in response to sodium selenite is limited. This work was performed to investigate the effects of exogenous sodium selenite application in promoting drought tolerance of cucumber. The drought tolerance of cucumber seedlings is enhanced under the application of selenite, positively influencing shoot fresh weight and chlorophyll relative content and altering the chloroplast ultrastructure. The contents of JA and JA-isoleucine (JA-ILE) increased significantly in response to selenite application under drought conditions. Furthermore, the expression of JA biosynthesis and regulatory genes, namely, LOX (Lipoxygenase), AOC (allene oxide cyclase), AOS (allene oxide synthase), and MYC2 (the basic helix-loop-helix (bHLH) protein) was upregulated to greater levels when selenite was added in combination with drought treatment. This study provides methods to mitigate drought stress and valuable theoretical support for further understanding the plant response to drought signals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium (Se) plays vital roles not only in human health by reducing the risks of cancer (Bañuelos et al. 2011; Radomska et al. 2021) and COVID-19 (Hargreaves and Mantle 2021; Mal'tseva et al. 2022) but also in plants resistance by enhancing tolerance to cold, heat, cadmium and water deficit stress (Huang et al. 2018; Wen 2021; Yang et al. 2021). Bioenhancement with Se can be effectively realized by adding Se to growing horticultural crops. The simultaneous biofortification of horticultural crops with Se and the improvement in plant stress tolerance is beneficial for both growers and consumers. Due to increasing interest in the functions of Se in horticultural crops, research into the effects of Se in crops has markedly increased recently. The application of Se modifies the biochemical responses of plants under stress caused by the early blight fungal phytopathogen Alternaria solani (Quiterio-Gutiérrez et al. 2019), increases the cold tolerance of cucumber seedlings (Yang et al. 2021), regulates plant antioxidant activity and leads to osmotic adjustment in plants under drought conditions (Dar et al. 2021). Selenium-treated potato show greater PSII efficiency of energy conversion and higher effective quantum yield (Germ et al. 2007). Through the glutathione peroxidase pathway, the application of Se increases the activity of antioxidant enzymes in response to abiotic stress (Lanza and Reis 2021). Recently, many studies have investigated the effect of Se in alleviating water deficiency stress (Ahmad et al. 2021; Zahedi et al. 2020), but further research is still needed for the specific mechanism.
Jasmonic acid (JA) is an important and widely distributed plant hormone that has essential functions in the processes of plant growth, development and defense (Song et al. 2014). Cis-12-oxo-phytodienoic acid (OPDA) is an early biosynthetic precursor of JA, and OPDA formation requires expression of the enzymes lipoxygenase (LOX) (Royo et al. 1996), allene oxide synthase (AOS) (Kuroda et al. 2005; Laudert et al. 1996) and allene oxide cyclase (AOC) (Riemann et al. 2013). The biosynthesis of JA from OPDA mainly depends on five enzymes, namely, OPDA reductase (OPR) (Biesgen and Weiler 1999; Stintzi and Browse 2000), OPC-8: CoA ligase (OPCL) (Kienow et al. 2008), acyl-CoA oxidase (ACX) (Oaxaca-Castillo et al. 2007; Vanhooren et al. 1997), multifunctional protein 2 (MFP2) (Richmond and Bleecker 1999) and peroxisomal acetyl-CoA acyltransferase 1 (PAAR) (Hayashi et al. 1998; Li et al. 2021; Sordillo et al. 2011). JASMONATE ZIM-domain (JAZ) repressors are recruited by CORONATINE INSENSITIVE 1 (COI1)-based SCF complexes in response to the JA signal for the ubiquitination, degradation and regulation of their downstream signaling components in various JA responses (Katsir et al. 2008; Thines et al. 2007; Xie et al. 1998). rd22BP1, renamed MYC2 (the basic helix-loop-helix (bHLH) protein), is a transcription factor that regulates genes involved in JA synthesis and metabolism (Abe et al. 2003). JA regulates the response to multiple abiotic stresses (Hu et al. 2017; Ku et al. 2018). Although studies have shown that JA participates in plant drought tolerance (Avramova 2019; Wasternack and Song 2017), little is known about the mechanism by which JA is involved in enhancing the drought tolerance under the application of selenite.
In this study, we demonstrated that JA participates in enhancing the drought tolerance under the application of Se in cucumber seedlings. Liquid chromatography–tandem mass spectrometry (LC–MS/MS) was used to determine the content of JA and JA derivatives. The whole gene transcriptome was characterized, and real-time quantitative PCR analysis was conducted to monitor gene expression. Levels of expression of the JA biosynthesis LOX, AOS, AOC and the transcription factor MYC2 genes were quantified, and the results showed that the LOX (Csa7G449420), AOC (Csa5G366670), AOS (Csa2G360780) and MYC2 (Csa3G011620) expression levels were significantly upregulated in cucumber seedlings in response to the exogenous application of Se under drought stress. A better understanding of the signal transduction of JA and Se under drought conditions in horticultural crops is presented in this study.
Materials and methods
Materials and treatments
Cucumber (Cucumis sativus L. ‘Jinyan No.4’) plants were used as the study materials. The method used for promoting germination and raising seedlings has been described in the literature (Yang et al. 2021). The substrate used for seedling growth consisted mainly of turf and vermiculite purchased from Jinan Baidouble Biological Technology Co., Ltd. Hoagland’s nutrient solution was applied to the vermiculite medium to support seedling growth. After 35 days of culture, seedlings at similar growth stages were well watered and then four different test treatments were applied: Control, with normal water and fertilizer management; T1, apply 1 μM sodium selenite under normal conditions; T2, natural drought treatment; and T3, apply selenite under drought treatment. Sodium selenite was applied via irrigation with water and fertilizer. The amount applied was enough to saturate the soil water. The control received the same amount of water and fertilizer. Twelve cucumber seedlings were included in each treatment group, and 3 representative seedlings were photographed.
Determination of plant growth index
The fresh shoot weights were measured after five days of treatment, and the chlorophyll relative content was measured based on the third or fourth leaf from the apical meristem after five days of treatment using the SPAD-502 Plus meter (Konica Minolta Sensing, JAPAN), with three replicates for each treatment.
Transmission electron microscopy analysis
A transmission electron microscopy analysis of chloroplast ultrastructure was carried out as previously described (Fukuda et al. 2013).
Analysis of ROS accumulation
The histochemical staining of superoxide anion (O2·−) was performed using nitroblue tetrazolium (NBT) (Xia et al. 2009).
Determination of antioxidant enzymatic activity
A sample (300 mg fresh weight) of cucumber leaves was used for the determination of antioxidant enzymatic activities (Yang et al. 2021). Superoxide dismutase (SOD) activity was assayed as previously described (Stewart and Bewley 1980). Catalase (CAT) activity was measured according to the method based on the decrease in the H2O2 content at 240 nm (Chamnongpol et al. 1996). Ascorbate peroxidase (APX) activity was measured based on the absorbance at 290 nm (Durner and Klessig 1995). Peroxidase (POD) activity was measured based on the absorbance at 470 nm (Nickel and Cunningham 1969).
Quantification of JA and JA derivatives
A fresh plant leaf sample was harvested, immediately frozen in liquid nitrogen, ground into powder (30 Hz, 1 min) and stored at −80°C. The quantification of endogenous JAs was performed by Wuhan Metware Biotechnology Co., Ltd. (Wuhan, China) using an LC–MS/MS platform as previously described (Guo et al. 2021).
Relative expression of genes related to JA biosynthesis and metabolism
The third leaf from the growing point of the seedlings was used to evaluate gene expression. RNA was isolated, cDNA libraries were multiplexed and sequenced, RNA-seq was performed, and clean reads were mapped by Novogene Co., Ltd. (Tianjin, China). Transcript reconstruction, clean read mapping, and gene expression calculation were performed according to previously described methods (Langmead and Salzberg 2012; Li and Dewey 2011; Pertea et al. 2015). The fragments per kilobase of transcript per million mapped reads (FPKM) values were used to estimate the data for each transcript. Relative gene expression was illustrated using TBtools software (Chen et al. 2020).
Real-time quantitative PCR was performed as previously described (Pfaffl 2001; Yang et al. 2021). The primers of LOX (Csa7G449420; F: GAGGTGATGGGAAAGGAGAAAG and R: CTTGGGCGAGCGTATTCTATT), AOC (Csa5G366670; F: CGTCTCAACTACCTCCAACAA and R: GTTCTTGTACTCTTGCGGATTTG), AOS (Csa2G360780; F: TCCTCGTCTTCTTCCTCTCTTC and R: AACATCGGTGGCCCATAATC), MYC2 (Csa3G011620; F: GAGACAACGGCGAGAGAAAT and R: GTCACCTAGTAGTGAGGCTTTG), JAZ (Csa3G645940; F: GACGAAAGTCTCGAGCGATTTA and R: TTCACTTGGTATGGACCTCTTG), JAZ (Csa7G448810; F: TTTCCTTCGTCGGGATTCTTC and R: CCGGCGTAGAATATGGTCATT) and ACTIN (Csa6G484600; F: TTCTGGTGATGGTGTGAGTC and R: GGCAGTGGTGGTGAACATG) were used. The primer search method was implemented by using the tomato genome, Gcorn plant, cucumber genome and Arabidopsis genome website (Yang et al. 2021).
Statistical analysis of the data
Microsoft Excel 2010 software was used to plot the data. The data are presented as the means ± standard deviations of three replicates. SAS software was used to carry out the statistical analyses by analysis of variance (ANOVA).
Results
Response of cucumber seedlings to exogenous Se under drought stress
The application of 1 μM sodium selenite enhanced the drought tolerance in terms of the cucumber seedling phenotype, shoot fresh weight and chlorophyll relative content (Fig. 1). Se application also increased plant height and individual leaf size, as revealed by the phenotype images in this figure. Under drought conditions, plant growth was inhibited, leaves wilted, the density of villi on the leaf surface increased and the color of the leaf is deeper compared with the control seedlings. In seedlings under the application of Se and drought stress, only the older leaves wilted, in contrast to seedlings under drought stress, in which both the older and younger leaves wilted. Compared with seedlings under drought stress, there are more unwilted leaves in seedlings under the application of Se and drought stress. The shoot weight of seedlings under the application of Se and drought stress was significantly higher than the shoot weight of seedlings under drought stress.
Effects of exogenous selenite application on cucumber seedlings under drought stress. (a) Phenotypes of cucumber seedlings under control (Control), under the application of Se (T1), under drought stress (T2) and under the application of Se and drought conditions (T3). (b) Shoot fresh weight of cucumber seedlings under Control, T1, T2 and T3 conditions. (c) Chlorophyll relative content of cucumber seedlings under Control, T1, T2 and T3 conditions. Values are expressed as the means of three replicates ± SDs. Samples with different letters are significantly different (P < 0.05). FW, fresh weight
Effects of exogenous Se application on plant photosynthetic apparatus
The leaf color, compared to the control, was deeper under the application of Se and drought stress (T3), and was deepest under drought stress (T2). To clarify this phenotype, the chloroplast ultrastructure of cucumber leaves in seedlings under different treatments was compared. Compared with the control, the amount of starch grains increased significantly uner the application of Se (T1) or under drought stress (T2), although the color of starch grains in seedlings under the application of Se were brighter without (T1) or with drought stress(T3), and the color of starch grains under drought stress (T2) were darker (Fig. 2). Under drought stress, the number of plastoglobules significantly increased relative to the control.
Exogenous application of selenite protected the plant photosynthetic apparatus in response to drought stress. The ultrastructure of cucumber seedling leaf cells under control (Control), under the application of Se (T1), under drought stress (T2) and under the application of Se and drought stress (T3). The red arrow indicates starch grains, and the blue arrow indicates plastoglobuli
Effects of exogenous application of Se on the oxidative stress induced by drought stress
Superoxide (O2·−) ions in leaves were observed by NBT staining, indicating that under drought stress conditions, reactive oxygen species (ROS) levels significantly increased compared with the control. However, the application of Se decreased the O2·− levels and increased the ROS-scavenging capability in leaves of cucumber seedlings under drought stress (Fig. 3a). Under control conditions, enzyme activity increased in response to exogenous selenite application but decreased under drought stress. Compared with drought treatment alone, the enzyme activities related to ROS-scavenging showed higher levels under selenite treatment in drought conditions (Fig. 3b).
Exogenous selenite application alleviated oxidative stress under drought conditions. (a) Superoxide (O2·.−) ions in cucumber leaves were detected by nitroblue tetrazolium (NBT) staining. (b) The activity levels of superoxide dismutase (SOD), peroxidase (POD), catalase (CAT) and ascorbate peroxidase (APX) of the control, T1, T2, and T3 treatments. Values are expressed as the means of three replicates ± SD. Any two samples within a figure with different letters are significantly different (P < 0.05)
Effects of Exogenous Selenite on Endogenous JA Content
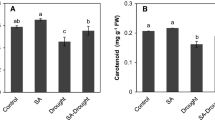
To determine whether JA participates in Se-induced drought tolerance of cucumber seedlings, liquid chromatography–tandem mass spectrometry (LC–MS/MS) (SCIEX, Framingham, MA, USA) was used. Compared with the control, the JA content decreased nonsignificantly in response to Se treatment, under normal condition whereas increased significantly in drought and Se-treated drought (in particular) conditions (Fig. 4a). Compared with the control, JA-isoleucine (JA-ILE) contents significant increased in response to drought and Se-treated drought conditions (Fig. 4b). The proportion of JA was the lowest in the T3 treatment (Fig. 4d).
Effects of exogenous selenite application on jasmonic acid (JA) and its derivatives. (a) JA content in cucumber seedling leaves under control, Se application (T1), drought stress (T2) and Se application and drought (T3) stress. (b) JA-isoleucine (JA-ILE) content in cucumber seedling leaves. Values are expressed as the mean of three replicates ± SD, and two samples within a figure with different letters are significantly different, according to ANOVA and Tukey’s test (P < 0.05). (c) Contents of JAs (JA, JA-ILE, JA-valine (JA-Val), JA-phenylalanine (JA-Phe), and MeJA (methyl jasmonate)) in cucumber seedling leaves. (d) The ratio of JA to other JAs (JA-ILE, JA-Val, JA-Phe, MeJA) in cucumber seedling leaves. FW, fresh weight
It was demonstrated that JA participated in Se-induced drought tolerance. Genes related to JA biosynthesis and regulation (transcription factors) were analyzed at the whole transcriptome level by quantifying the genes' expression (Fig. 5). The expression of LOX (Csa7G449420), AOC (Csa5G366670), AOS (Csa2G360780) and MYC2 (Csa3G011620) was significantly upregulated in the cucumber seedlings under the application of Se and drought stress compared with those under drought stress alone. These results suggest that the four genes LOX (Csa7G449420), AOC (Csa5G366670), AOS (Csa2G360780) and MYC2 (Csa3G011620) might be important for the increase in total JA content. The expression of JAZ (Csa3G645940, Csa7G448810 and Csa1G597690) also increased under T3 treatment compared with the drought stress alone.
Relative expression genes involved in jasmonic acid (JA) biosynthesis, metabolism and transcription factors with exogenous selenite treatment under drought stress. The whole transcriptome was analyzed to quantify the relative expression of genes involved in JA synthesis, derivation and degradation, as well as genes encoding JA-related repressors and transcription factors. C, control, T1, under the application of selenium, T2, under the drought stress and T3, under the application of Se and drought stress. Red reflects the normalization value of the fragments per kilobase of transcript per million (FPKM): the higher the value, the deeper the color. LOX, lipoxygenase; AOC, allene oxide cyclase; AOS, allene oxide synthase; OPR, Cis-12-oxo-phytodienoic acid reductase; OPCL, OPC-8: CoA ligase; ACX, acyl-CoA oxidase; MFP2, multifunctional protein 2; PAAR, peroxisomal acetyl-CoA acyltransferase 1; JAR, jasmonoyl-isoleucine synthetase; COI, coronatine insensitive; JAZ, jasmonate zim-domain; MYC2, the basic helix-loop-helix (bHLH) protein, a transcription factors; OPDA, Cis-12-oxo-phytodienoic acid
Discussion
The beneficial effect of Se in increasing plant adaptation to unfavorable stresses has been reported (Yang et al. 2021), with several studies indicating that Se alleviates drought stress in different crops, such as rice (Luo et al. 2021), strawberry (Zahedi et al. 2020), mung bean (Aqib et al. 2021), camelina (Camelina sativa L.) and canola (Brassica napus L.) (Ahmad et al. 2021). Our former research indicated that 1 μM Se treatment was the optimal treatment which was been used in the present study (Yang et al. 2021). Our study demonstrated that exogenous selenite application effectively induced drought tolerance in cucumber seedlings, reducing the inhibition of shoot fresh weight under drought conditions (Fig. 1b). Furthermore, the application of Se significantly reduced the deepening of leaf color (as SPAD value indicates) under drought treatment compared with the control (Fig. 1c). Photosynthesis determines the accumulation of plant fresh weight. Chlorophyll is a vital pigment for photosynthesis in plants, and the SPAD value is an important indicator of relative chlorophyll content. Furthermore, the chloroplast ultrastructure, determined using transmission electron microscopy, revealed the impacts of drought and Se (Fig. 2).
There were two main findings from this part of the study. First, drought significantly promoted an increase in the number of starch grains and the accumulation of starch. The detailed chloroplasts analysis indicated that the starch grains from the drought treatment were darker than those from the condition under the application of Se and drought stress. This variation possibly reflects differences in starch composition, and the content and proportions of straight-chain amylose and branched-chain amylopectin in starch. The second finding is that the application of Se and drought stress effectively attenuated the increase in the number of plastoglobules observed under drought stress. Plastoglobules are involved in the disintegration of thylakoids in chloroplasts, and the number of plastoglobules increased under drought stress, whereas the application of Se and drought stress attenuated the drought-induced increase.
Damage to the chloroplast structure easily leads to the production of photoelectrons during photosynthesis and an increase of ROS levels. Superoxide ions were detected by NBT staining, and the content increased markedly in the drought treatment compared with the control (Fig. 4a). The results indicated that the application of Se and drought stress effectively increased antioxidant enzyme activity and reduced ROS accumulation (Fig. 4b). Therefore, the application of Se under drought treatment reduced ROS-induced cell damage and promoted shoot weight accumulation.
Se application significantly increased the JA content, especially under drought stress (Fig. 5). Several studies have reported that JA content responds to abiotic stresses (Ali and Baek 2020; Awan et al. 2021; Gupta et al. 2021; Wang et al. 2020; Xing et al. 2020). Selenium application increased the total JA content under drought conditions while decreasing the ratio of JA to JA derivatives under drought stress, which suggested that Se promoted the transformation of JA to its derivatives.
The expression of the genes LOX (Csa7G449420), AOC (Csa5G366670), AOS (Csa2G360780) and MYC2 (Csa3G011620) significantly increased in cucumber seedlings under the application of Se and drought stress compared with those under the drought stress (Fig. 5), indicating that these genes might be crucial in regulating the increase in the total content of JA in cucumber seedlings. The expression of JAZ (Csa3G645940, Csa7G448810 and Csa1G597690) also increased in seedlings under the Se application with drought treatment compared with those under the drought treatment. These results suggest a possible negative feedback regulatory mechanism to limit the increase in JA content and regulate the balance of plant hormones.
A mechanism diagram was developed based on this study (Fig. 6). The expression of JA biosynthesis genes (LOX, AOS and AOC) was induced by exogenous Se application, regulating the biosynthesis of JA. The application of Se with drought treatment inhibited ROS accumulation and thus decreased chlorophyll damage, further increasing drought stress tolerance.
Schematic illustration of jasmonic acid (JA) participating in inducing drought tolerance by exogenous application of Se to cucumber seedlings. Expression of JA biosynthesis genes was induced by exogenous application of selenium, which regulated the biosynthesis of JA. Selenium application also inhibited reactive oxygen species (ROS) accumulation and decreased chlorophyll degradation, further increasing drought stress tolerance. LOX, lipoxygenase; AOC, allene oxide cyclase; AOS, allene oxide synthase
Conclusion
Se significantly enhances plant resistance to stress. This study demonstrated that Se effectively increased the plant chlorophyll content under drought stress, maintained the chloroplast integrity, promoted the accumulation of starch and other dry matter in chloroplasts, improved the antioxidant capacity, and reduced the damage caused by reactive oxygen species. JA was shown to be involved in selenium-enhanced drought tolerance in cucumber seedlings, and Se promoted the production of JA and its derivatives and improved the conversion of JA to its derivatives. The key Se-induced genes involved in JA synthesis, transformation and regulation under drought stress were identified to provide theoretical support for exogenous regulation and plant tolerance breeding.
Availability of data and materials
The datasets during and/or analysed during the current study available from the corresponding author on reasonable request.
References
Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell. 2003;15:63–78. https://doi.org/10.1105/tpc.006130.
Ahmad Z, Anjum S, Skalicky M, Waraich EA, Muhammad Sabir Tariq R, Ayub MA, et al. Selenium Alleviates the Adverse Effect of Drought in Oilseed Crops Camelina (Camelina sativa L.) and Canola (Brassica napus L.). Molecules. 2021;26:1699. https://doi.org/10.3390/molecules26061699.
Ali MS, Baek KH. Jasmonic Acid Signaling Pathway in Response to Abiotic Stresses in Plants. Int J Mol Sci. 2020;21:621. https://doi.org/10.3390/ijms21020621.
Aqib M, Nawaz F, Majeed S, Ghaffar A, Ahmad KS, Shehzad MA, et al. Physiological insights into sulfate and selenium interaction to improve drought tolerance in mung bean. Physiol Mol Biol Plants. 2021;27:1073–87. https://doi.org/10.1007/s12298-021-00992-6.
Avramova Z. Defence-related priming and responses to recurring drought: Two manifestations of plant transcriptional memory mediated by the ABA and JA signalling pathways. Plant Cell Environ. 2019;42:983–97. https://doi.org/10.1111/pce.13458.
Awan SA, Khan I, Rizwan M, Zhang X, Brestic M, Khan A, et al. Exogenous abscisic acid and jasmonic acid restrain polyethylene glycol-induced drought by improving the growth and antioxidative enzyme activities in pearl millet. Physiol Plant. 2021;172:809–19. https://doi.org/10.1111/ppl.13247.
Bañuelos GS, Fakra SC, Walse SS, Marcus MA, Yang SI, Pickering IJ, et al. Selenium accumulation, distribution, and speciation in spineless prickly pear cactus: a drought- and salt-tolerant, selenium-enriched nutraceutical fruit crop for biofortified foods. Plant Physiol. 2011;155:315–27. https://doi.org/10.1104/pp.110.162867.
Biesgen C, Weiler EW. Structure and regulation of OPR1 and OPR2, two closely related genes encoding 12-oxophytodienoic acid-10,11-reductases from Arabidopsis thaliana. Planta. 1999;208:155–65. https://doi.org/10.1007/s004250050545.
Chamnongpol S, Willekens H, Langebartels C, Van Montagu M, Inzé D, Van Camp W. Transgenic tobacco with a reduced catalase activity develops necrotic lesions and induces pathogenesis-related expression under high light. Plant J. 1996;10:491–503. https://doi.org/10.1046/j.1365-313X.1996.10030491.x.
Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, et al. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020;13:1194–202. https://doi.org/10.1016/j.molp.2020.06.009.
Dar ZM, Malik MA, Aziz MA, Masood A, Dar AR, Hussan SU, et al. Role of selenium in regulation of plant antioxidants, chlorophyll retention and osmotic adjustment under drought conditions: a review. Int J Plant Soil Sci. 2021;33:52–60. https://doi.org/10.9734/IJPSS/2021/V33I1430504.
Durner J, Klessig DF. Inhibition of ascorbate peroxidase by salicylic acid and 2,6-dichloroisonicotinic acid, two inducers of plant defense responses. Proc Natl Acad Sci U S A. 1995;92:11312–6. https://doi.org/10.1073/pnas.92.24.11312.
Fukuda M, Wen L, Satoh-Cruz M, Kawagoe Y, Nagamura Y, Okita TW, et al. A guanine nucleotide exchange factor for Rab5 proteins is essential for intracellular transport of the proglutelin from the Golgi apparatus to the protein storage vacuole in rice endosperm. Plant Physiol. 2013;162:663–74. https://doi.org/10.1104/pp.113.217869.
Germ M, Kreft I, Stibilj V, Urbanc-Berčič O. Combined effects of selenium and drought on photosynthesis and mitochondrial respiration in potato. Plant Physiol Bioch. 2007;45:162–7. https://doi.org/10.1016/j.plaphy.2007.01.009.
Guo Q, Li X, Niu L, Jameson PE, Zhou W. Transcription-associated metabolomic adjustments in maize occur during combined drought and cold stress. Plant Physiol. 2021;186:677–95. https://doi.org/10.1093/plphys/kiab050.
Gupta A, Bhardwaj M, Tran LP. JASMONATE ZIM-DOMAIN family proteins: important nodes in jasmonic acid-abscisic acid crosstalk for regulating plant response to drought. Curr Protein Pept Sci. 2021;22:759–66. https://doi.org/10.2174/1389203722666211018114443.
Hargreaves IR, Mantle D. COVID-19, Coenzyme Q10 and Selenium. Adv Exp Med Biol. 2021;1327:161–8. https://doi.org/10.1007/978-3-030-71697-4_13.
Hayashi M, Toriyama K, Kondo M, Nishimura M. 2,4-Dichlorophenoxybutyric acid-resistant mutants of Arabidopsis have defects in glyoxysomal fatty acid β-oxidation. Plant Cell. 1998;10:183–95. https://doi.org/10.1105/tpc.10.2.183.
Hu Y, Jiang Y, Han X, Wang H, Pan J, Yu D. Jasmonate regulates leaf senescence and tolerance to cold stress: crosstalk with other phytohormones. J Exp Bot. 2017;68:1361–9. https://doi.org/10.1093/jxb/erx004.
Huang C, Qin N, Sun L, Yu M, Hu W, Qi Z. Selenium improves physiological parameters and alleviates oxidative stress in strawberry seedlings under low-temperature stress. Int J Mol Sci. 2018;19:1913. https://doi.org/10.3390/ijms19071913.
Katsir L, Chung HS, Koo AJ, Howe GA. Jasmonate signaling: a conserved mechanism of hormone sensing. Curr Opin Plant Biol. 2008;11:428–35. https://doi.org/10.1016/j.pbi.2008.05.004.
Kienow L, Schneider K, Bartsch M, Stuible HP, Weng H, Miersch O, et al. Jasmonates meet fatty acids: functional analysis of a new acyl-coenzyme A synthetase family from Arabidopsis thaliana. J Exp Bot. 2008;59:403–19. https://doi.org/10.1093/jxb/erm325.
Ku YS, Sintaha M, Cheung MY, Lam HM. Plant hormone signaling crosstalks between biotic and abiotic stress responses. Int J Mol Sci. 2018;19:3206. https://doi.org/10.3390/ijms19103206.
Kuroda H, Oshima T, Kaneda H, Takashio M. Identification and functional analyses of two cDNAs that encode fatty acid 9-/13-hydroperoxide lyase (CYP74C) in rice. Biosci Biotech Bioch. 2005;69:1545–54. https://doi.org/10.1271/bbb.69.1545.
Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–9. https://doi.org/10.1038/nmeth.1923.
Lanza MGDB, dos Reis AR. Roles of selenium in mineral plant nutrition: ROS scavenging responses against abiotic stresses. Plant Physiol Bioch. 2021;164:27–43. https://doi.org/10.1016/j.plaphy.2021.04.026.
Laudert D, Pfannschmidt U, Lottspeich F, Holländer-Czytko H, Weiler EW. Cloning, molecular and functional characterization of Arabidopsis thaliana allene oxide synthase (CYP 74), the first enzyme of the octadecanoid pathway to jasmonates. Plant Mol Biol. 1996;31:323–35. https://doi.org/10.1007/BF00021793.
Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. https://doi.org/10.1186/1471-2105-12-323.
Li M, Yu G, Cao C, Liu P. Metabolism, signaling, and transport of jasmonates. Plant Commun. 2021;2:100231. https://doi.org/10.1016/j.xplc.2021.100231.
Luo H, Xing P, Liu J, Pan S, Tang X, Duan M. Selenium improved antioxidant response and photosynthesis in fragrant rice (Oryza sativa L.) seedlings during drought stress. Physiol Mol Biol Plants. 2021;27:2849–58. https://doi.org/10.1007/s12298-021-01117-9.
Mal’tseva VN, Goltyaev MV, Turovsky EA, Varlamova EG. Immunomodulatory and anti-inflammatory properties of selenium-containing agents: their role in the regulation of defense mechanisms against COVID-19. Int J Mol Sci. 2022;23:2630. https://doi.org/10.3390/ijms23042360.
Nickel KS, Cunningham BA. Improved peroxidase assay method using leuco 2,3’,6-trichloroindophenol and application to comparative measurements of peroxidatic catalysis. Anal Biochem. 1969;27:292–9. https://doi.org/10.1016/0003-2697(69)90035-9.
Oaxaca-Castillo D, Andreoletti P, Vluggens A, Yu S, van Veldhoven PP, Reddy JK, et al. Biochemical characterization of two functional human liver acyl-CoA oxidase isoforms 1a and 1b encoded by a single gene. Biochem Bioph Res Co. 2007;360:314–9. https://doi.org/10.1016/j.bbrc.2007.06.059.
Pertea M, Pertea GM, Antonescu CM, Chang TC, Mendell JT, Salzberg SL. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol. 2015;33:290–5. https://doi.org/10.1038/nbt.3122.
Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. https://doi.org/10.1093/nar/29.9.e45.
Quiterio-Gutiérrez T, Ortega-Ortiz H, Cadenas-Pliego G, Hernández-Fuentes AD, Sandoval-Rangel A, Benavides-Mendoza A, et al. The application of selenium and copper nanoparticles modifies the biochemical responses of tomato plants under stress by Alternaria solani. Int J Mol Sci. 2019;20:1950. https://doi.org/10.3390/ijms20081950.
Radomska D, Czarnomysy R, Radomski D, Bielawski K. Selenium compounds as novel potential anticancer agents. Int J Mol Sci. 2021;22:1009. https://doi.org/10.3390/ijms22031009.
Richmond TA, Bleecker AB. A defect in β-oxidation causes abnormal inflorescence development in Arabidopsis. Plant Cell. 1999;11:1911–24. https://doi.org/10.1105/tpc.11.10.1911.
Riemann M, Haga K, Shimizu T, Okada K, Ando S, Mochizuki S, et al. Identification of rice Allene Oxide Cyclase mutants and the function of jasmonate for defence against Magnaporthe oryzae. Plant J. 2013;74:226–38. https://doi.org/10.1111/tpj.12115.
Royo J, Vancanneyt G, Pérez AG, Sanz C, Störmann K, Rosahl S, et al. Characterization of three potato lipoxygenases with distinct enzymatic activities and different organ-specific and wound-regulated expression patterns. J Biol Chem. 1996;271:21012–9. https://doi.org/10.1074/jbc.271.35.21012.
Song S, Qi T, Wasternack C, Xie D. Jasmonate signaling and crosstalk with gibberellin and ethylene. Curr Opin Plant Biol. 2014;21:112–9. https://doi.org/10.1016/j.pbi.2014.07.005.
Sordillo JE, Sharma S, Poon A, Lasky-Su J, Belanger K, Milton DK, et al. Effects of endotoxin exposure on childhood asthma risk are modified by a genetic polymorphism in ACAA1. BMC Med Genet. 2011;12:158. https://doi.org/10.1186/1471-2350-12-158.
Stewart RRC, Bewley JD. Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol. 1980;65:245–8. https://doi.org/10.1104/pp.65.2.245.
Stintzi A, Browse J. The Arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate synthesis. Proc Natl Acad Sci U S A. 2000;97:10625–30. https://doi.org/10.1073/pnas.190264497.
Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, et al. JAZ repressor proteins are targets of the SCFCOI1 complex during jasmonate signalling. Nature. 2007;448:661–5. https://doi.org/10.1038/nature05960.
Vanhooren JCT, Marynen P, Mannaerts GP, Van Veldhoven PP. Evidence for the existence of a pristanoyl-CoA oxidase gene in man. Biochem J. 1997;325:593–9. https://doi.org/10.1042/bj3250593.
Wang J, Song L, Gong X, Xu J, Li M. Functions of jasmonic acid in plant regulation and response to abiotic stress. Int J Mol Sci. 2020;21:1446. https://doi.org/10.3390/ijms21041446.
Wasternack C, Song S. Jasmonates: biosynthesis, metabolism, and signaling by proteins activating and repressing transcription. J Exp Bot. 2017;68:1303–21. https://doi.org/10.1093/jxb/erw443.
Wen D. Selenium in horticultural crops. Sci Hortic. 2021;289:110441. https://doi.org/10.1016/j.scienta.2021.110441.
Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG. COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science. 1998;280:1091–4. https://doi.org/10.1126/science.280.5366.1091.
Xing Q, Liao J, Cao S, Li M, Lv T, Qi H. CmLOX10 positively regulates drought tolerance through jasmonic acid-mediated stomatal closure in oriental melon (Cucumis melo var. makuwa Makino). Sci Rep. 2020;10:17452. https://doi.org/10.1038/s41598-020-74550-7.
Yang N, Sun K, Wang X, Wang K, Kong X, Gao J, et al. Melatonin participates in selenium-enhanced cold tolerance of cucumber seedlings. Front Plant Sci. 2021;12:786043. https://doi.org/10.3389/fpls.2021.786043.
Zahedi SM, Moharrami F, Sarikhani S, Padervand M. Selenium and silica nanostructure-based recovery of strawberry plants subjected to drought stress. Sci Rep. 2020;10:17672. https://doi.org/10.1038/s41598-020-74273-9.
Acknowledgements
We appreciate International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Funding
This research was supported by the Agriculture Industrial Technology System Funding of Shandong Province of China (SDAIT-05), the Shandong Provincial Key Research and Development Program (2021TZXD007-02), the Agricultural Scientific and Technological Innovation Project of Shandong Academy of Agricultural Sciences (CXGC2022A06, CXGC2022A23, CXGC2023A19, CXGC2023A34), the Key Joint Tackling of Scientific and Technological Cooperation between Shandong and Chongqing (cstc2019jscx-lyggX0002).
Author information
Authors and Affiliations
Contributions
Conceptualization, DW and NY; methodology, YH; software, XW; resources, JS; writing—original draft preparation, NY; writing—review and editing, DW and YZ; visualization, SS; project administration, XW; funding acquisition, NY. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wen, D., Zheng, Y., Han, Y. et al. Sodium selenite increases drought tolerance by promoting jasmonic acid biosynthesis in cucumber. HORTIC. ADV. 1, 6 (2023). https://doi.org/10.1007/s44281-023-00009-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44281-023-00009-0